Abstract
Chromium (VI) is a well-known toxic, industrial, water pollutant which has various, adverse effects on environmental health. Utilization of agricultural waste in effluent water treatment would minimize the problem of water pollution. The present study deals with the use of three types of nut shells for Cr(VI) removal. Adsorbents are characterized, using point of zero charges (pHpzc), FTIR, BET surface area analysis, and SEM. The variation of different operating parameters on metal removal was conducted. The best sorption kinetic model was pseudo-second order. The adsorption process is both physical and chemical, and this depends on temperature. The Cr(VI) adsorption is spontaneous and endothermic. According to isotherm studies, Langmuir isotherm model fits fairly well for all adsorbents. Regeneration studies suggest that the adsorbents have proper regeneration criteria and can be used for multiple times. Study on RBC count of Gallus gallus domesticus gives concrete evidence of deadly effects of Cr(VI). It also figures out that the effluent solution treated with bio-adsorbents is less harmful. The scale-up design procedure is reported here. This study proved that groundnut shell, walnut shell, and almond shell have immense potential and can be utilized even after regeneration as replacement of commercial adsorbents for industrial wastewater. GA–ANN modeling has been developed for the best possible wastewater treatment management.
Similar content being viewed by others
1 Introduction
Industrial wastes which are produced from textile, painting, leather, tanning, paper, cement, steel, and others get discharged into water bodies. This is harmful without any prior treatment given to the natural water bodies [1]. Chromium (VI) is the most toxic water pollutant among these heavy metals; it has six oxidation states; among them, hexavalent and trivalent are the most stable state. The high toxicity of Cr(VI) is a major concern as Cr(III) is relatively insoluble in water. Chromium (VI) has toxic effects on both animals and humans and is also a well-known carcinogen [2]. Longtime exposure to Cr(VI) causes dermatitis, ulcerations, and allergic skin reactions in the human body [3, 4].
To reduce water pollution, various treatment processes are practiced. Apart from adsorption, the other processes are precipitation, membrane separation, ion exchange, electrochemical precipitation, reverse osmosis, nanofiltration, and others [5, 6]. These techniques have their advantages as well as drawbacks [7,8,9]. Sludge formation with its disposal is the main drawback of the precipitation process. High operating cost diminishes the use of ion exchange as a better alternative. Due to the higher operating cost of other methods, the adsorption process is extensively used as an efficient, simple, economic water treatment process [10, 11].
Variety of agricultural waste like coconut shell, rice husk, shells of wheat, sawdust, wheat straw, shells of rice, pistachio, walnut, peanut, and almond is used as low-cost bio-adsorbent [12,13,14]. The synthetic adsorbents are appealing with respect to their adsorption capacity but its high investment and preparation cost, hence, not suitable for rural industrial application, particularly in the Third World countries like India. So the green adsorbents, i.e., agricultural waste like nut shells, different leaves widely available in the rural areas without any cost, are the most suitable for adsorptive removal. The green adsorbent means natural adsorbents without any physical and chemical treatment [15]. Hence, in the batch mode Cr(VI) remotion from aqueous solution by the shells of the groundnut, walnut, and almond is used as low-cost green adsorbents for the experimental investigated. Applicability of different kinetic and isotherm models is tested [16, 17]. Desorption studies have been tested to check their reusability criteria. The toxicity difference of metal-containing solution before and after adsorption on red blood cells (RBCs) of Gallus gallus domesticus is also investigated. The scale-up design procedure is reported.
Researchers used different artificial neural network (ANN) and genetic algorithm (GA)–ANN hybrid modeling to solve engineering problems [18,19,20]. The advantage of GA–ANN modeling over the ANN is evident in the less computational time required and gives better predictability. Hence, in the present study [21], the model parameters are optimized by using GA and then Lavenberg–Marquardt algorithm is used for the final prediction.
2 Experimental methodology
Groundnut, walnut, and almond shells are obtained from the local market, washed, and then dried at 105 °C temperature for 24 h. After this, dried shells are ground and sieved to prepare a size of 250–350 µm.
Merck analytical grade chemicals are used. The metal-stock solution of 1000 ppm is prepared by adding 2.8286 gm K2Cr2O7 in 1000 ml distilled water. By diluting it, the desired solution is prepared.
The equipment used is as follows:
-
1.
WTW pH meter (Multi 340i/SET, Germany)
-
2.
BET (Quantachrome, Novawin 1000, USA)
-
3.
FTIR (IS5Nicholet ATR, Thermo Fischer Scientific, USA)
-
4.
UV–visible spectrophotometer (DR-5000 spectrophotometer, Hach, USA)
-
5.
SEM (ZEISS, EVO-Q400 ++, Germany)
2.1 Experimental
Detailed of the experiment is given in our earlier paper [22, 23]. The Cr(VI) ion is analyzed by UV–visible spectrophotometer using 1,5 diphenyl carbazide coloring reagent at 540 nm [15, 16]. The amount of Cr(VI) adsorbed, and the removal percentage was calculated as,
Experiments were repeated thrice with reproducibility and relative deviation within the range of ± 0.5% and ± 2.5%, respectively.
3 Results and discussion
3.1 Adsorbent characterization
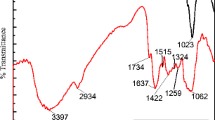
Table 1 shows the results of FTIR analysis. It indicates that there are various activities, functional groups in fresh and Cr(VI)-loaded adsorbents. The existence of a broad peak indicates that phenolic or alcoholic –OH group is present in the adsorbents. This group involves in Cr(VI) adsorption as the peak of groundnut shell moves from 3310 to 3302 cm−1, the peak of almond shell shifts from 3324 to 3331 cm−1, and peak of walnut shell shifts from 3326 to 3300 cm−1 after adsorption. The frequency around 3310 cm−1 shows the existence of phenolic or alcoholic –OH group and shifts from 3310 to 3302 cm−1 indicating the chromium binding with (–OH) groups. At pH 1 or 2, H+ ion neutralizes the (–OH) groups on the surface bearing and forms –OH2+ site that enhances the HCrO4− ion to adsorb. The aliphatic C–H stretching group is involved in adsorption of Cr(VI) as the peak moves from 2923 to 2920 cm−1 for groundnut shell, the peak of almond shell shifts from 2921 to 2934 cm−1 while the peak of walnut shell shifts from 2903 to 2899 cm−1. The aldehyde C–H stretching is also responsible for Cr(VI) adsorption for groundnut shell as the peak shifts from 2851 to 2854 cm−1. Aliphatic acid C=O stretching takes part in Cr(VI) adsorption for groundnut shell and almond shell as the peak shifts from 1713 to 1734 cm−1 and 1735 to 1717 cm−1, respectively. Alkene groups (C=C stretching) also interfere in the adsorption process, because peaks are shifted from 1620 to 1652 cm−1 (groundnut shell), 1636 to 1650 cm−1 (almond shell), 1607 to 1598 cm−1 (walnut shell) after adsorption. Aromatic groups (C=C stretching) on walnut shell peak move from 1509 to 1501 cm−1. Peaks at 1414, 1418, and 1371 cm−1 indicate the carboxylic acid groups (C–O stretching) in the groundnut shell, almond shell, and walnut shell, respectively, and it shifts 1363, 1421, and 1325 cm−1 after adsorption. Peaks at 1226, 1230 and 1240 cm−1 are for stretching of C–O that indicate ester groups which are present in adsorbents and shifted after Cr(VI) adsorption. The wavelength of 1023 cm−1 (groundnut) and 1019 cm−1 (almond) and 1028 cm−1 (walnut) is for deformation of glycosidic C–H with OH bending and ring vibration. It indicates that there is a β glycosidic bond between glucose and cellulose [15, 16]. The peaks at 897, 890, and 889 cm−1 in Cr(VI)-loaded groundnut, almond, and walnut shell are indicative of the presence of Cr=O or Cr–O vibration which is responsible for Cr(VI) adsorption in the adsorbent surface.
SEM (Scanning Electron Microscope) images of fresh and Cr(VI)-loaded adsorbents are represented in Figs. 1a, b, 2a, b, 3a, b groundnut, walnut, and almond shell, respectively. SEM images of fresh adsorbents showed that the surface is porous and irregular, whereas for Cr(VI)-loaded adsorbents the SEM images are less porous. Cr(VI) ion is having crystal radius of 0.52 Å and binds with the various active groups which, in turn, blocked the pores partially [15, 16].
The solid addition method is used to measure pHpzc of the adsorbents [11, 24]. 30 mL of KNO3 solutions (0.01 M) of different pH (3–10) was prepared. 0.1 g of bio-adsorbent was poured in each conical flask and equilibrated for the duration of 24 h. After that, the pH of supernatant solutions was measured. The intersection point between the initial pH and pH difference gives the value of pHpzc. The similar procedure was carried out using 0.1 M KNO3 solution. Adsorption of cations will be favorable at pH > pHpzc because, at this point, the surface of the adsorbents is negatively charged, which increases the electrostatic force of attraction. At lower pH (pH < pHPZC), the positively charged adsorbent surface attracts anions. pHpzc values are 6.21, 6.10, and 6.42 for groundnut, almond, and walnut shells, respectively.
The surface area of adsorbents is 1.83 m2/gm, 11.27 m2/gm, and 10.16 m2/gm for groundnut, almond, and walnut shell, respectively, and determined using Brunauer–Emmett–Teller (BET) analysis.
3.2 Variation of operating parameters
The optimum pH of influent Cr(VI) solution is to achieve maximum removal efficiency by different nut shells. The maximum remotion occurred at pH 2.0 for groundnut shell and walnut shell, whereas for the almond shell, it is found at pH 1.0 (Fig. 4). At low pH, the HCrO4− is predominant [10, 11]. At the acidic pH, large amount of H+ ions is located onto the surface of the adsorbent and it creates a strong, attractive electrostatic force between the chromate ions and absorbent. As the pH > 6.0 the concentration OH− ion increases and competes with CrO42− ions to reach the active sites.
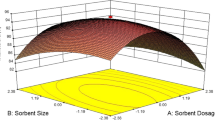
Effects of different adsorbent dosage were found by conducting experiments with varying amounts (0.1–8 gm/L). Figure 5 shows that the Cr(VI) remotion increases with the increasing dosages of the adsorbent. It is also shown from the figure that the remotion efficiency will remain the same after adsorbent dosages of 2.5 gm/L for groundnut shell and 1.5 gm/L for almond and walnut. The metal binding sites for adsorption increase with the increase in adsorption dosage, but it remains constant for the higher dosages of the adsorbent.
Figure 6 shows the change of the Cr(VI) adsorption during different contact time. Agitation time for this study was set for 4–5 h. Initial adsorption was rapid and reached equilibrium at a prolonged rate. The nature of varying nut shells and their available adsorption sites affect the magnitude of equilibrium time. At the beginning, the adsorption rate was rapid, and more than 80% were absorbed within 2 h for the walnut shell, and in the case for groundnut and almond shell, it took 4 h. After that, the rate of adsorption process decreases gradually and remains constant at equilibrium. Solution concentration will remain the same after reaching the equilibrium.
Figure 7 depicts the dependence of metal adsorption process with initial Cr(VI) concentrations (5–20 mg/L). The figure indicates as the increase in the initial metal ion concentration, the percentage of remotion decreases, whereas uptake capacity increases. At lower metal concentration, the numbers of available active sites on the adsorbent are much higher, but with the increase in initial metal ion concentration, the available active sites became saturated faster, hence decreasing the metal remotion percentage.
3.3 Kinetics study
3.3.1 Pseudo-first-order model
Lagergren pseudo-first-order model is [25],
The parameters are estimated by plotting of \(\ln \left( {q{}_{e} - q_{t} } \right)\) against t and listed in Table 2. The parameter k1 has no specific trend with the increase in initial Cr(VI) concentration for groundnut and almond shell, whereas for walnut shell k1 decreases as the Cr(VI) concentration increases. The R2 values are not very impressive for all the adsorbents [26].
3.3.2 Pseudo-second-order model
This model [26] is:
The kinetic parameters are obtained from the plot of \(\left( {\frac{t}{{q_{t} }}} \right)\) versus t and are shown in Table 2. The value of k2 reduces with the increase in initial Cr(VI) concentration, and high R2 value suggests that this model fits better for all adsorbents.
3.3.3 Elovich model
Second-order kinetics was described by the Elovich equation considering energetically heterogeneous solid surfaces [27]. But this model failed to explain the mechanism for adsorbate–adsorbent interaction. The linear model equation is as follows:
By plotting qt versus lnt, the different model parameters were determined and are shown in Table 2. As the metal ion concentration increases, the magnitude of \(a_{e}\) increases, whereas the \(b_{e}\) decreases for groundnut shell. For walnut shell as the Cr(VI) concentration increases, both the parameter value reduced, and for the almond shell, no specific trend was observed. According to the value of R2 and trend of parameters, application of Elovich model is significant for groundnut shell and walnut shell.
3.3.4 Fractional power model
Freundlich equation is modified to get the fractional power model [28] which can be described by the following linear equation:
Table 2 presents the value of both constants and other parameters. An increasing trend of K was noticed with the increase in initial Cr(VI) ion concentration for the ground nutshell, but the value of v has no specific trend which limits its applicability for groundnut shell. For walnut and almond shell, both the constants decrease with the increase in initial Cr(VI) concentration. The v is a positive constant less than unity and shows the time dependence of liquid-phase adsorption of Cr(VI) onto different nut shells. The value of K suggests the strength of the site for Cr(VI) binding. From Table 2, it can be observed that the strength of binding of Cr(VI) onto walnut shell is much stronger than the other nut shells used. The higher values of the correlation coefficient for all the three nutshells validate the appropriateness of fractional power.
3.3.5 Natarajan and Khalaf model
The kinetic equation of Natarajan and Khalaf model [29] is given as:
The value of kad is determined by plotting \(\log \left( {\frac{{C_{0} }}{{C_{t} }}} \right)\) versus t and is shown in Table 2 and observed that as the initial metal concentration increases, the value of kad decreases for all three adsorbents.
The heterogeneous reversible first-order kinetics is the main assumption of this model. It explains the relationship between the concentration of initial and at time t. The high values of correlation coefficient signify that the adsorption process depends on the concentration of Cr(VI) at the aqueous solution at any given time. The high values of correlation coefficient signify that the adsorption process depends on the concentration of Cr(VI) at the aqueous solution at any given time. The correlation coefficient value R2 for all adsorbents is less in comparison with pseudo-second-order model.
3.3.6 Intraparticle diffusion model
The adsorption process is a complex process with more than one rate-limiting step. Weber and Moris first explored the possibility of intraparticle diffusion-controlled adsorption process and developed a model equation for the same [30].
Equation parameters are shown in Table 2. The intraparticle diffusional rate constant increases with the increase in initial Cr(VI) concentration. Though the R2 value indicates the fitness of the equation, it has not passed through the origin; hence, it is not the rate-limiting step [30].
3.4 Rate-limiting step prediction
The adsorption mechanism has one or more than one rate-controlling mechanisms, just like chemical adsorption, film diffusion, or intraparticle diffusion of ions on the active sites of the surface. To illustrate Cr(VI) ion diffusion process on the pores of different bio-adsorbents, the equation of Fick’s was used [10]
\(q_{\alpha }\) is replaced by \(q_{e}\), and the plots of \(\frac{{q_{t} }}{{q_{e} }}\) versus t0.5 for groundnut shell, walnut shell, and almond shell depicted multi-linear segments and are presented in Fig. 8a–c. These figures indicated that more than one phenomenon governs the adsorption process. Even at the initial point of time, one factor may be rate controlling, which may change with time [31]. The plot of \(\frac{{q_{t} }}{{q_{e} }}\) versus t0.5 divided into three linear segments each of them represents the different mechanism of mass transfer. First linear segment represents film diffusion mass transfer, second one represents intraparticle diffusion, and the last linear segment represents adsorption–desorption equilibrium. For groundnut shell, film diffusion segment took 79 min, whereas intraparticle diffusion took 1 min. For the walnut shell, film diffusion segment took 70 min, whereas intraparticle diffusion took 4 min. For the almond shell, film diffusion segment took 71 min, whereas intraparticle diffusion took 3 min. These signify that film diffusion mass transfer controls the entire adsorption process; this may be due to the formation of the thin film.
3.5 Adsorption isotherm models
3.5.1 Langmuir isotherm
This model is based on the monolayer adsorption on the homogeneous surface having energetically equivalent sites [32], and the linearized form is
RL a dimensionless factor is expressed as [15]
RL values indicate that either the process is irreversible (RL = 0) or linear (RL = 1), favorable (0 < RL < 1) or unfavorable (RL > 1). Table 3 shows that qmax decreases as temperature increases for all the adsorbents, whereas the value of b increases for all the adsorbents. The values of RL indicate favorable adsorption for all applied conditions for all the adsorbents [33]. According to the value of R2 and the trend of parameters, application of the Langmuir model is highly significant for all the adsorbents.
3.5.2 Freundlich isotherm
It applies for multilayer adsorption and interaction of adsorbed molecules on the heterogeneous surface. This model considers an exponential decrease in sorption energy on adsorbent sorption centers completion [34]. Expression of this model is as follows:
Table 3 shows the parameter and both the value of Kf and n increase as the temperature increases for all adsorbents. The parameter n must lie between 1 and 10 for favorable adsorption, and Table 3 indicates favorable adsorption for all adsorbents.
3.5.3 Dubinin–Radushkevich isotherm
The isotherm equation [35, 36] is
Here ε is Dubinin–Radushkevich isotherm constant, and it has been also termed as Polanyi potential and is defined [37] by
The sorption energy E is estimated as
The value of E gives the nature of adsorption; for chemical adsorption, this value is 8 kJ/mol < E < 16 kJ/mol, and for physical adsorption, it is E < 8 kJ/mol. Table 3 indicates the physical adsorption for all adsorbents in the temperature 303 K and 313 K, but in the case of 323 K temperature, chemical adsorption occurs for all adsorbents.
3.5.4 Temkin isotherm
This isotherm [38] was based on the interaction of adsorbent–adsorbate and refers that with the increase in surface coverage, the heat of adsorption increases.
The constant B (J/mol) can be calculated from
Linear plot for Temkin adsorption isotherm gives values of AT, \(b_{\text{T}}\) and B and is presented in Table 3. AT and \(b_{\text{T}}\) values increase with the increase in temperature for all adsorbents, whereas the value of B decreases for the increase in temperature for all the adsorbents.
3.5.5 Harkins and Jura isotherm
This model that is originally applied for the gas–solid system is extended to solution–solid systems and assumed that condensed-type adsorbed films are formed on the adsorbent surface [39]. The isotherm is expressed as
As per Table 3, the value of AH has an increase for all the adsorbent with the increase in temperature, whereas BH decreases for the walnut shell. But no specific trends were found for the other two adsorbents. According to the correlation coefficient data, this model is not suitable for the Cr(VI) adsorption onto the nut shells.
From the statistical point of view, the Langmuir isotherm was the best-fitted isotherm for all the adsorbents used.
3.6 Thermodynamic studies
Three different temperatures (30, 40 and 50 °C) were chosen to describe the influence of temperature on Cr(VI) adsorption. The results show that the adsorption capacity of all three adsorbents is increased with the increase in temperature; hence, the adsorption process is facilitated by a higher temperature. The thermodynamic behavior [40] of the sorption was calculated from the following equations
and
where the value of the thermodynamic equilibrium constant can be obtained from the apparent equilibrium constant, \(K_{c}^{/}\) at different temperature and initial concentration of Cr(VI) for each adsorbent system and extrapolating to zero [10] where
Table 4 shows the thermodynamic parameters as calculated. The negative value of ΔG° for all adsorbents indicates the spontaneous nature of Cr(VI) adsorption. Further, it decreases with the increase in temperature and shows that the degree of spontaneity increases at a higher temperature. The ΔG° value showed ranges between 0 and − 20 kJ/mol which indicates the physisorption process [41]. Positive values of ΔS° reflect a increase in the degree of disorderliness at the adsorption interface during the adsorption of Cr(VI). The endothermic nature of the adsorption process by various nutshells was confirmed by the positive values of ΔH° [10, 42].
3.7 Mechanism of adsorption
The nature of nut shell’s surface, different functional sites and their interaction behavior with adsorbing molecule govern the mechanism of adsorption. The FTIR spectra of fresh nut shells and Cr(VI) adsorbed nut shells suggest the possible interaction of Cr(VI) ion with phenolic or alcoholic –OH group. The spectral differences between the beginning of adsorption and after the adsorption suggest that the aliphatic C–H stretching and the aliphatic acid C=O stretching might take part in the adsorption process. The adsorbent pHpzc value is important as it detects the ionic state of the adsorbent.
In this study, maximum adsorption occurs at lower pH 1 or 2 for the nut shells and is less than the pHpzc. Hence, the active sites of the all the nut shells are charged positive [10, 11]. At low pH, chromium present in HCrO4− form binds with the positively charged active groups by electrostatic attraction forces. The strength of electrostatic and hydrogen bonding interaction has been further confirmed from the result of Temkin and D–R isotherm models. D–R isotherm also indicates the governing of physical adsorption process in the temperature 303 K and 313 K, while at the 323 K temperature it shifts toward a chemical adsorption process for all the adsorbents. The weak interaction between Cr(VI) and the adsorbents can further be confirmed from the regeneration studies of different nut shells by changing the pH of the solution. The film diffusion is the main mass transfer process for Cr(VI) remotion in the nut shells.
3.8 Regeneration studies
Regeneration capability decides the usefulness of an adsorbent because multiple uses of adsorbent reduce the operational cost. NaOH solutions of different concentrations (0.1–0.75 M) used for desorption of Cr(VI) are presented in Table 5. This table indicates that as the concentration of NaOH solution increases, desorption of Cr(VI) also increases, which suggest that Cr(VI) adsorption is chemical [40]. Regeneration of the adsorbents was done up to second cycle, and Cr(VI) removal capacity after each desorption cycle is shown in Table 6 which depicts that with the successful regeneration of the adsorbents, the adsorption capacity decreases.
3.9 Comparison of the adsorptive capacities
The experimental adsorption capacity was compared with the other previously reported natural adsorbents and presented in Table 7. Adsorptive capacities of all these shells are quite good and comparable with others.
3.10 Effects on biological indicator (RBC)
3.10.1 Blood collection and separation of red blood cell (RBC)
Chicken blood sample was collected from Govt. recognized slaughter house (Chitpur Slaughter House, 400, Borough-II, B.K Paul Avenue, Rabindra Sarani, Garanhatta, Beniatola, Kolkata-700005, West Bengal, India). To prevent coagulation of collected blood sample from adult chicken (Gallus gallus domesticus), 2% EDTA solution was used [63]. Caution was taken while collecting the blood samples. Before the separation of RBC, the blood samples were cleaned by adding 0.9% saline solution in a centrifuge for 10 min at 1000 rpm. This procedure was repeated until the collection of fresh RBC.
3.10.2 Study of RBC
Three milliliters of RBC suspension was exposed to 500 µL of synthetic Cr(VI) solution of 20 mg/L concentration. In other batches, 3 ml of RBC suspension was exposed to 500 µL of different adsorbent-treated Cr(VI) solutions, which is collected from the adsorption process at time intervals of 20, 60, and 120 min. After this, RBC suspensions were incubated for 1 h at 37 °C for further analysis. After the incubation period, RBC suspensions were washed three times. Then, slides are viewed and counted in the optical microscope (93309, Olympus microscope, Hemocytometer, India). The microscopic count at a different time interval of treatment is shown in Fig. 9.
3.10.3 Toxicity
In Fig. 9, untreated Cr(VI) solution showed decreasing RBC count after 1-h exposure as compared to the control. This result indicates that untreated Cr(VI) solution gives enough stress to the RBC. Application of treated Cr(VI) solution shows improvement in red blood cells count. As the increase in treatment time Cr(VI) percentage in solution decreases, which in turn recover the deficiency in RBC count.
4 Scale-up design
The mass balance equation for the adsorption process is [17]
When the system reaches equilibrium, the equation became
The best-fitted isotherm model for all the three systems is the Langmuir isotherm model, and this was used for design purpose. By combining the Langmuir isotherm equation with the mass balance equation, the following is obtained
From the sets of known values of b and qmax for the individual bio-adsorbents, the equation was further simplified, and a relationship for \(\frac{W}{V}\) was obtained by using C0 and Ce. Figure 10 shows the mass of walnut shell required to remove Cr(VI) ion at the initial concentration of 20 mg/L. Table 8 shows the adsorbent amount required for 80% remotion of Cr(VI).
5 Modeling of the network
Genetic algorithm (GA) is one of the techniques of optimization used in some cases during the last decade [20]. The use of a neural network to prune the choice of inputs is well documented in the past [63]. The use of the Levenberg–Marquardt algorithm (with the maximum of 1000 iterations and the possible application of stopping criterion within 100 iterations for improvement) for this purpose (optimize the number of nodes in the hidden layer within the value of 1–25) is also well documented. The choices of data that are not normalized are now a usual practice and are demonstrated in the past in our previous efforts [20, 21]. Therefore, this analysis is performed with the use of original data. The inputs of the GA are presented in Table 8 along with their range. Apart from the adsorbent number, the measurable quantities, e.g., pH, adsorbent dosage, contact time, temperature, and initial metal ion concentration, are considered. The predictable parameter, i.e., the output, is the percentage removal. For the elimination of the random error, the data have been independently randomized three times and are subsequently analyzed three times [63], and the predicted data are recorded. The division of data related to training, cross-validation, and the predicted output is divided into the usual 70, 20, and 10 percent, respectively, for the present study.
The roulette selection rule, the 1 point crossing overrule (crossover probability of 0.9), and the uniform mutation rule (mutation probability of 0.1) have been used. For the present analysis, the size of the population is chosen as 15 and 100 generations have been set for the evolution of the present GA network.
5.1 Network performance
Figure 11 shows the change in the MSE (minimum value of cross-validation) to that of the various generations for the three different randomizations. The optimized network can be considered keeping in mind the smallest numerical value achieved relating to the minimum value MSE for cross-validation during one of the generation numbers. Table 9 presents this MSE numerical value.
Table 10 represents the final performance of output regarding the statistical error parameters. The excellence of the performance is determined from the numeric value of the error parameters presented in Table 10. The observation yields the numerical value of the cross-correlation coefficient (R) to be exceeding the lower limit of 0.97 to the upper limit 0.997. This alone presents the effectiveness of the modeling. This observation can also be ratified by the numerical value of the AARE and SD. The visual inspection of Fig. 12 indicates that the closeness of the output to the experimental data reiterates the excellent performance of the GA modeling (Table 10).
6 Conclusions
In this present study, groundnut shell, walnut shell, and almond shells were utilized to remove Cr(VI) in batch mode. The results of this investigation indicate that Cr(VI) adsorption depends on solution pH. Optimum pH for groundnut and walnut shell is 2, and for the almond, the shell is 1. The Cr(VI) removal efficiency of natural bio-adsorbents is quite high and is strongly dependent on operational parameters. The adsorption capacities of the nut shells are found to be 22.94 mg/g, 24.52 mg/g, and 24.25 mg/g, for the groundnut, walnut, and almond shells, respectively. Among the applied kinetic models, the pseudo-second order fits well for all three adsorbents. The prediction of rate-limiting steps reveals that film diffusion controls the Cr(VI) adsorption process for all three adsorbents. The Langmuir isotherm describes better process equilibrium than others. Dubinin–Radushkevich model predicts that Cr(VI) adsorption on groundnut shell, walnut shell, and almond shell are governed by both physical and chemical adsorption depending on temperature. The adsorption process has also been found to be spontaneous and endothermic. The regeneration of the bio-adsorbents also finds its reusability as an adsorbent. Study of effects on RBC also gives a supporting proof of toxicity of Cr(VI) and the applicability of the used adsorbents for Cr(VI) removal. The scale-up design is also described. GA–ANN modeling successfully predicts metal removal. Lastly, easy availability with high adsorption capacity and good recovery make all of these three green bio-adsorbents a potential alternatives for the removal of toxic Cr(VI) ion from wastewater.
Abbreviations
- A H :
-
Harkins–Jura isotherm constant
- A T :
-
Equilibrium binding constant of Temkin isotherm (L/g)
- a e :
-
Adsorption rate (initial) (mg/(g min))
- B :
-
Heat of adsorption (J/mol)
- B H :
-
Harkins–Jura isotherm constant
- b :
-
Constant of Langmuir model (L/mg)
- b e :
-
Chemisorption activation energy (g/mg)
- b T :
-
Constant of Temkin isotherm
- C :
-
External convective mass transfer (mg/g)
- C a :
-
Cr(VI) ion concentration at the adsorbent at equilibrium (mg/L)
- C 0 :
-
Cr(VI) ion concentration (initial) (mg/L)
- C e :
-
Cr(VI) ion concentration at equilibrium (mg/L)
- C t :
-
Ion concentration of Cr(VI) at time t (mg/L)
- D e :
-
Absorbate’s effective diffusion coefficient in the absorbent phase (m2/s)
- E :
-
Adsorption free energy (KJ/mol)
- ΔG 0 :
-
Gibbs free energy change (kJ/mol)
- ΔH 0 :
-
Enthalpy change (kJ/mol)
- K :
-
Fractional power model constant (mg/g)
- k 1 :
-
Rate constant of Lagergren model (min−1)
- k 2 :
-
Rate constant of pseudo-second-order model (g/mg min)
- K ad :
-
Rate constant of Natarajan and Khalaf model (min−1)
- K f :
-
Constant of Freundlich model (mg/g)/(mg/L)1/n
- k i :
-
Rate constant of intraparticle diffusion model (mg/(g min0.5))
- \(K_{c}^{0}\) :
-
Thermodynamic equilibrium constant
- \(K_{c}^{/}\) :
-
Apparent equilibrium constant
- n :
-
Factor of heterogeneity
- q e :
-
Sorption capacity at equilibrium (mg/g)
- q max :
-
Adsorption capacity of Langmuir isotherm, mg/g
- q s :
-
Theoretical isotherm saturation capacity (mg/g)
- q t :
-
Sorption capacity at time t (mg/g)
- R :
-
Ideal gas constant (J/(mol K))
- R 2 :
-
Correlation coefficient
- R a :
-
Adsorbent particle radius (m)
- R L :
-
Dimensionless factor
- ΔS 0 :
-
Change of entropy (kJ/mol K)
- T :
-
Temperature (K)
- t :
-
Time (min)
- V :
-
Solution volume (L)
- v :
-
Fractional power model constant (min−1)
- W :
-
Adsorbent mass (g)
- X m :
-
Maximum adsorption capacity of adsorbent (mmol/g)
- λ :
-
Constant related to sorption energy (mol2/kJ2)
- ε :
-
Polanyi potential (kJ2/mol2)
References
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Biores Technol 99:6709–6724
Govind P, Madhuri S (2014) Heavy metals causing toxicity in animals and fishes. Res J Anim Vet Fish Sci 2(2):17–23
Koren HS (1995) Associations between criteria air pollutants and asthma. Environ Health Perspect 103(Suppl 6):235–242
Hu Q, Xiao Z, Xiong X, Zhou G, Guan X (2015) Predicting heavy metals’ adsorption edges and adsorption isotherms on MnO2 with the parameters determined from Langmuir kinetics. J Environ Sci (China) 27:207–216
Ozer A, Altundogan HS, Erdem M, Tumen F (1997) A study on the Cr(VI) removal from aqueous solutions by steel wool. Environ Poll 97:107–112
Namasivayam C, Yamuna RT (1995) Adsorption of chromium (VI) by a low-cost adsorbent: biogas residual slurry. Chemosphere 30:561–578
Padilla AP, Tavani EL (1999) Treatment of an industrial effluent by reverse osmosis. Desalination 126:219–226
Rengaraj S, Joo CK, Kim Y, Yi J (2003) Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J Hazard Mater 102:257–275
Ahmed MT, Taha S, Chaabane T, Akretche D, Maachi R, Dorange G (2006) Nanofiltration process applied to the tannery solutions. Desalination 200:419–420
Singha B, Das SK (2011) Biosorption of Cr(VI) ions from aqueous solutions: kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf B Biointerfaces 84:221–232
Banerjee M, Bar N, Basu RK, Das SK (2017) Comparative study of adsorptive removal of Cr(VI) ion from aqueous solution in fixed bed column by peanut shell and almond shell using empirical models and ANN. Environ Sci Pollut Res 24:10604–10620
Dupont L, Guillon E (2003) Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ Sci Technol 37:4235–4241
Singha B, Das SK (2013) Adsorptive removal of Cu (II) from aqueous solution and industrial effluent using natural/agricultural wastes. Colloids Surf B Biointerfaces 107:97–106
Siddiqui Sharf I, Chaudhry Saif A (2018) Nigella sativa plant based nanocomposite-MnFe2O4/BC: an antibacterial material for water purification. Environ Res 200:996–1008
Banerjee M, Bar N, Basu RK, Das SK (2018) Removal of Cr(VI) from its aqueous solution using green adsorbent pistachio shell: a fixed bed column study and GA–ANN modeling. Water Conserv Sci Eng 3(1):19–31
Banerjee M, Basu RK, Das SK (2018) Cr(VI) adsorption by a green adsorbent walnut shell: adsorption studies, regeneration studies, scale-up design and economic feasibility. Proc Safe Environ Prot 116:693–702
Banerjee M, Basu RK, Das SK (2019) Cu(II) removal using green adsorbents: kinetic modeling and plant scale-up design. Environ Sci Pollut Res 26(12):11542–11557
Bar N, Biswas MN, Das SK (2010) Prediction of pressure drop using artificial neural network for gas non-Newtonian liquid flow through piping components. Ind Eng Chem Res 49(19):9423–9429
Singha B, Bar N, Das SK (2014) The use of artificial neural networks (ANN) for modeling of adsorption of Cr(VI) ions. Des Water Treat 52(1–3):415–525
Ghaedi M, Daneshfar A, Ahmadi A, Momeni MS (2015) Artificial neural network-genetic algorithm based optimization for the adsorption of phenol red (PR) onto gold and titanium dioxide nanoparticles loaded on activated carbon. J Ind Eng Chem 21:587–598
Bahrami S, Ardejani FD, Baafi E (2016) Application of artificial neural network coupled with genetic algorithm and simulated annealing to solve groundwater inflow problem to an advancing openpit mine. J Hydrol 536:471–484
Naiya TK, Bhattacharya AK, Das SK (2008) Adsorption of Pb(II) by sawdust and neem bark from aqueous solutions. Environ Prog 27(3):313–328
Ilyas M, Ahmed A, Saeed M (2013) Removal of Cr(VI) from aqueous solutions using peanut shell as adsorbent. J Chem Soc Pak 35(3):760–768
Srivastava VC, Swamy MM, Mall ID, Prasad B, Mishra IM (2006) Adsorptive removal of phenol by bagasse fly ash and activated carbon: equilibrium, kinetics and thermodynamics. Colloids Surf A Physicochem Eng Asp 272:89–104
Lagergren S (1898) Zur theorie der sogenannten adsorption gelӧster stoffle, Kungliga Sevenska Vetenskapasakdemiens. Handilinger 24(4):1–39
Siddiqui Sharf I, Chaudhry Saif A (2019) Nanohybrid composite Fe2O3-ZrO2/BC for inhibiting the growth of bacteria and adsorptive removal of arsenic and dyes from water. J Clean Prod 223:849–868
Rudzinski W, Panczyk T (2002) The Langmuirian adsorption kinetics revised: a farewell to the XXth century theories? Adsorption 8:23–34
Venckatesh R, Amudha T, Sivaraj R, Chandramohan M, Jambulingam M (2010) Kinetics and equilibrium studies of adsorption of direct Red-28 onto Punica granatum Carbon. Int J Eng Sci Technol 2(6):2040–2050
Shahul Hameed K, Sivakumar S, Satheesh Kannan R (2016) Isotherm and kinetic studies on the adsorption of Commassie Brilliant Blue on commercial activated carbon and kaolin. Global J Adv Res 3:723–731
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanitary Eng Div 89:31–60
Ho YS (2003) Removal of copper ions from aqueous solution by tree fern. Water Res 37:2323–2330
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(1918):1361–1403
Singha B, Das SK (2011) Biosorption of Cr(VI) ions from aqueous solutions: kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf B Biointerfaces 84(1):221–232
Freundlich HMF (1906) Über die adsorption in losungen. Z Phys Chem 57(A):385–470
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266
Dubinin MM, Zaverina ED, Radushkevich LV (1947) Sorption and structure of active carbons I. Adsorption of organic vapors. Zh Fiz Khim 21:1351–1362
Polanyi M (1932) Section III—theories of the adsorption of gases. A general survey and some additional remarks. Trans Faraday Soc 28:316–333
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327–356
Harkins WD, Jura G (1943) An absolute method for the determination of the area of a fine crystalline powder. J Chem Phys 11:430
Bhattacharya AK, Naiya TK, Mandal SN, Das SK (2008) Adsorption kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J 137(3):529–541
Siddiqui Sharf I, Chaudhry Saif A (2018) A review on graphene oxide and its composites preparation and their use for the removal of As3+ and As5+ from water under the effect of various parameters: application of isotherm, kinetic and thermodynamics. Process Saf Environ Protect 119:138–163
Siddiqui Sharf I, Chaudhry Saif A (2019) Nigella sativa seed based nanocomposite-MnO2/BC: an antibacterial material for photocatalytic degradation, and adsorptive removal of dye from water. Environ Res 171:328–340
Sharma DC, Foster CF (1994) A preliminary examination into the adsorption of hexavalent chromium using low-cost adsorbents. Bioresour Technol 47:257–264
Donmez GC, Aksu Z, Ozturk A, Kutsal T (1999) A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem 34:885–892
Wang XS, Chen LF, Li FY, Chen KL, Wan WY, Tang YJ (2010) Removal of Cr(VI) with wheat-residue derived black carbon: reaction mechanism and adsorption performance. J Hazard Mater 175:816–822
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res 34:2955–2962
Hamadi NK, Chen XD, Farid MM, Lu MGQ (2001) Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chem Eng J 84:95–105
Selvi K, Pattabhi S, Kadirvelu K (2001) Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour Technol 80:87–89
Low KS, Lee CK, Low CH (2001) Sorption of chromium (VI) by spent grain under batch conditions. J Appl Polym Sci 82:2128–2134
Aoyama M, Tsuda M (2001) Removal of Cr(VI) from aqueous solutions by larch bark. Wood Sci Technol 35(5):425–434
Dakiky M, Khamis M, Manassra A, Mer’eb M (2002) Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res 6:533–540
Yu LJ, Shukla SS, Dorris KL, Shukla A, Margrave JL (2003) Adsorption of chromium from aqueous solutions by maple sawdust. J Hazard Mater B 100:53–63
Acar FN, Malkoc E (2004) The removal of chromium(VI) from aqueous solutions by Fagus orientalis L. Biores Technol 94(1):13–15
Romero-González Peralta-Videa JR, Rodriiguez E, Ramirez SL, Gardea-Torresdey JL (2005) Determination of thermodynamic parameters of Cr(VI) adsorption from aqueous solution onto Agave lechuguilla biomass. J ChemThermodyn 37:343–347
Sarin V, Pant KK (2006) Removal of chromium from industrial waste by using eucalyptus bark. Biores Technol 97:15–20
Malkoc E, Nuhoglu Y (2007) Determination of kinetic and equilibrium parameters of the batch adsorption of Cr(VI) onto waste acorn of Quercusithaburensis. Chem Eng Process 46:1020–1029
Gao H, Liu Y, Zeng G, Xu W, Li T, Xia W (2008) Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste rice straw. J Hazar Mater 150(2):446–452
Gonzalez MH, Georgia L, Claudia BP, Eveline M (2008) Coconut coir as biosorbent for Cr(VI) removal from laboratory wastewater. J Hazar Mater 159(2–3):252–256
Nag S, Mondal A, Bar N, Das SK (2017) Bio-sorption of chromium (VI) from aqueous solutions and ANN modeling. Environ Sci Poll Res 24:18817–18835
Pakade VE, Ntuli TD, Ofomaja AE (2017) Biosorption of hexavalent chromium from aqueous solutions by macadamia nutshell powder. Appl Water Sci 7:3015–3030
Chwastowski J, Staroń P, Kołoczek H, Banach M (2017) Adsorption of hexavalent chromium from aqueous solutions using Canadian peat and coconut fiber. J Mol Liq 248:981–989
Fernanda PF, Tannous K, Coppi CC (2019) Biosorption of hexavalent chromium from aqueous solutions using raw coconut fiber as a natural adsorbent. Chem Eng Commun 1:1. https://doi.org/10.1080/00986445.2018.1557154
Nag S, Mondal A, Nath Roy A, Bar N, Das SK (2018) Sustainable bioremediation of Cd(II) from aqueous solution using natural waste materials: kinetics, equilibrium, thermodynamics, toxicity studies and GA–ANN hybrid modeling. Environ Technol Innov 11:83–104
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, A., Banerjee, M., Bar, N. et al. Adsorptive removal of Cr(VI) from aqueous solution: kinetic, isotherm, thermodynamics, toxicity, scale-up design, and GA modeling. SN Appl. Sci. 1, 776 (2019). https://doi.org/10.1007/s42452-019-0813-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0813-9