Abstract
Chalcopyrite (CuFeS2) is commonly used ore in production of copper, but leaching of this ore is very slow and inefficient due to “passivation” during leaching at atmospheric conditions. In this study, in order to overcome drawbacks of the passivation layers, the concentrate supplied from Eti Bakır A.Ş. Küre Plant in Turkey was roasted at 600 °C for 1 h and after leached. Box–Wilson procedure of statistical experimental design was utilized to identify the effects of significant leaching variables for instance leaching time (X1; 10–120 min), solid/liquid ratio (X2; 0.01–0.20), and H2SO4 concentration (X3; 0.01–1.00 M) on Cu extraction (%) from roasted concentrate and was tried to be optimized. The coefficients of response functions have been calculated by regression analysis, and the estimates have been found to be well in line with the experimental outcomes. The optimal leaching parameters, time, solid/liquid rate and H2SO4 concentration were determined as 115 min, 0.116, and 0.71 M, respectively, and the highest Cu extraction (%) value was calculated as 92.45%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Copper, one of the most important metals for humanity for more than five thousand years [1, 2], is still among the most common metal used in the industry [3,4,5]. In nature, copper is generally found in the form of sulfide and oxide minerals such as azurite, malachite, tenorite, chrysocolla, bornite, brochantite, enargite, chalcopyrite, chalcocite, and covellite [3,4,5,6,7,8]. The most utilized mineral for the copper production [1, 2], chalcopyrite (CuFeS2), is an important and plentiful source that accounts for about 70% of all copper in the world, and is one of the widest spread copper-bearing minerals [9,10,11,12,13,14].
The copper extraction from chalcopyrite (CuFeS2) is performed by using hydrometallurgical and pyrometallurgical processes. About 80–85% of the copper production in the world is executed with the traditional pyrometallurgical processes, which consist of concentration by flotation, smelting, fire refining, and electro-refining routes [1, 2, 11, 12, 15,16,17,18,19]. Recently, due to technical and economic reasons of the traditional pyrometallurgical process [20] such as high-grade ore requirement [21], high investment for smelting and refining plants, as well as undesirable environmental effects caused by the formation of sulfur oxides (SO2 and/or SO3) [17, 19, 21,22,23], the hydrometallurgical process becomes an alternative route to copper extraction from low grade and complex sulfide copper concentrates [17, 20, 24,25,26,27,28,29]. Copper extraction from chalcopyrite by hydrometallurgical methods can be classified according to used type of solvent such as chloride/chlorine systems, nitric acid, sulfuric acid, ammoniacal solution and biological systems [19, 30,31,32]. In many studies in the literature, sulfuric acid and hydrochloric acid are the most preferred for leaching in acidic environments [32,33,34,35,36]. It is stated in the literature that the consumption of sulfuric acid, which is the cheapest solvent for leaching of oxidized copper ores [37], varies between 0.4 and 0.7 tons H2SO4 per ton of copper recovered, depending on the nature of the ore [36].
Despite the advantages of the hydrometallurgical process such as short construction time, low cost, operation simplicity, typically less energy-intensive, more suitable for low grade and complex ore types, and lower environmental impacts [18, 38,39,40,41,42], the extraction of copper from chalcopyrite in acidic medium is both very slow and ineffective at low temperatures due to the stable passivation layers [11, 17, 19, 22, 33,34,35, 42,43,44,45,46,47,48]. It has been stated in the literature that grinding to a very fine particle size [49], pressure, temperature, powerful oxidizing agents [7, 19, 32, 36, 50,51,52,53,54,55,56,57,58], and roasting/calcination of the ore/concentrate [19, 30, 59] can be used to increase the dissolution kinetic and copper extraction recovery by eliminating the effect of this passivation layer forms, whose chemistry, nature, and formation mechanism are still poorly understood [11, 15, 32, 42, 60,61,62].
Roasting is the key unit operation in converting low solubility sulfide ores into high solubility oxides. During roasting, chalcopyrite reacts with air and decomposes to generate copper sulfate, sulfur dioxide, sulfur, copper-iron oxides, copper-oxy sulfate, and copper ferrite [63, 64]. In between 577 and 667 °C temperature range, it is stated that the roasting reactions Eq. (1) occurring as follows and CuSO4 is formed, and above 745 °C, CuFe2O4 is formed according to the following reaction Eq. (2) [19, 30, 65,66,67]. It is stated that SO2, which is a problem in the roasting process as well as the pyrometallurgical processes and which can be converted into sulfuric acid normally [18, 19], can also be neutralized by using CaO according to the reaction Eq. (3) given below [68, 69]. In our study, our products obtained after roasting are in the form of CuFe2O4 (Cu Ferrite) and Cu4O5. CuFe2O4 phase seen in the mineralogical analysis is CuO.Fe2O3. Fe2O3 is not soluble in H2SO4 solutions. Cu4O5 is a metastable phase, and it comprises of CuO and Cu2O. Possible dissolution reaction suggestions for CuFe2O4 (Cu Ferrite; CuO.Fe2O3), CuO and Cu2O in H2SO4 solution were added as Eqs. (4 and 5) [70,71,72,73].
Significant copper resources in Turkey are scattered in the north of the country, and Küre copper ore deposit is one of the most important ones. Some studies have been carried out on leaching of Küre chalcopyrite concentrate using with some lixiviants [74,75,76,77,78]. However, it has not been used in roasted the concentrate leaching in previous studies. The aim of this study was to determine the suitability of the roasted Küre Chalcopyrite Concentrate for H2SO4 leaching after roasting and to produce data on the evaluation of domestic chalcopyrite ore/concentrate. For this purpose, the effects of leaching time, solid/liquid ratio, and H2SO4 concentration which are important parameters in roasted chalcopyrite leaching on Cu extraction (%) were examined by using Box–Wilson experimental design and optimum parameters were determined.
2 Materials and methods
2.1 Materials
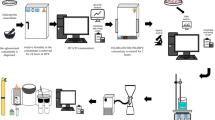
Chalcopyrite concentrate samples were supplied from Küre Copper Concentrator Plant in Kastamonu, Turkey (Eti Bakır A.Ş.), flotation concentration plant. It was sieved to -212 µm before dried at 105 °C for 12 h. The mineralogical analysis results of the chalcopyrite concentrate samples are given in Table 1, and the mineralogical analysis results of the roasted concentrate, which has been subjected to oxidizing roasting process at 600 °C for 1 h according to the data in the literature [6, 19, 20, 42, 63, 65, 67, 79,80,81,82,83], are given in Table 2.
The chalcopyrite concentrates samples were roasted by using Protherm brand MoS-B 180/8 Model (1.800 °C) Muffle Furnace in Hitit University Faculty of Engineering Department of Metallurgical and Materials Engineering. X-Ray Diffraction (XRD) analysis was carried out by using the PANalytical brand X’Pert Pro model XRD Device in İstanbul Technical University Faculty of Engineering Department of Metallurgical and Materials Engineering Laboratories. XRF analysis was carried out by using Thermo Scientific brand Niton™ XL3t 950 GOLDD + model XRF Spectrometer in Cumhuriyet University Faculty of Engineering Department of Metallurgical and Materials Engineering. Solutions of H2SO4 (Sigma-Aldrich) were used as lixiviant. All chemicals used were at least analytical grade.
2.2 Methods
The experiments were performed within the scope of the study using the Box–Wilson experimental design, and optimization was made for the parameters effective in the extraction. In this context, independent parameters in the Box–Wilson experimental design method were used; time: 10–120 min, solid/liquid ratio: 0.01–0.20, and H2SO4 concentration: 0.01–1.00 M.
The experiments were employed in a 400-mL beaker placed in a temperature-controlled water bath and sealed with a watch glass. Heidolph MR Hei-Tec model a magnetic stirrer having a digital controller unit, timer and thermostat was used for effective mixing and heating. A magnetic stirrer provided the agitation (400 rpm) required for homogeneity. Mono-distilled water (pH ≅ 6.5, approximately) was employed in the leaching experiments. After the required leaching temperature (60 °C) was achieved, the amount of chalcopyrite required for the calculated solid/liquid ratio (0.01–0.20) was added to the 200 mL solution containing water and H2SO4, required for calculated H2SO4 concentration (0.01–1.00 M). At the end of the desired leaching time (10–120 min), the suspension was filtered and the solid residues were thoroughly washed, dried, and the amount of copper in the filtrate was identified by XRF analysis. In this study, the Cu extraction (%) values, which are a measure of how much of the total copper is leached, were calculated using Eq. (6) given below:
2.2.1 Box–Wilson experimental design
Details of the Box–Wilson statistical experimental design procedure can be found in the literature [84, 85]. Three working variables, i.e., time, solid/liquid ratio and the H2SO4 concentration, were chosen as the most significant independent variables. The time (X1) was changed between 10 and 120 min; the solid/liquid ratio (X2) between 0.01 and 0.20; and the H2SO4 concentration (X3) between 0.01 and 1.00. The experimental design comprised of six axial points (A), eight factorial points (F), and three center points (C). For estimating the experimental error calculation, the center point was repeated three times. The experimental terms as coded values and real values utilized for the Box–Wilson statistical design are given in Table 3.
The Cu extraction (Y) was correlated with the other independent variables (X1, X2, X3) using a quadratic regression. Design Expert 8.0 was used to determine Eq. (7) coefficients by regression analysis of the experimental data.
3 Results and discussion
A comparison of the values of empiric and predicted for the Cu extraction (%) is summarized in Table 4. The detected Cu extraction (%) values varied between 42.13% and 90.11%. The determination coefficient (R2 values) between the observed and predicted value was 0.9959 for Cu extraction (%), indicating well in line between the values detected and those predicted.
3.1 The effects of time and solid/liquid ratio
The variation of the Cu extraction (%) in a fixed 0.505 M of H2SO4 concentration depending on time and solid/liquid ratio is given in Fig. 1.
As seen from Fig. 1, at the time rise up to 50 min, the Cu extraction (%) declined with increasing solid/liquid ratio, and reached a minimum value in the maximum solid/liquid ratio. In between 0.05 and 1.6 solid/liquid ratio range, the Cu extraction (%) values started to increase again with increasing time above 50 min and the Cu extraction (%) reached its maximum value in about 120 min. However, the Cu extraction (%) values rapidly decreased both at low (less than 0.5) and high (over than 1.6) solid/liquid ratio, at time above 50 min. At medium solid/liquid ratio values, increasing Cu extraction (%) due to the increase in time over 50 min can be attributed to the increased interaction of the particles with H2SO4 solutions [86]. At low leaching time (especially under 50 min), the decrease in Cu extraction (%) due to the increase in the solid/liquid ratio may be caused by the increase in the amount of particles per unit volume of the lixiviant [63, 69, 87, 88], worsening of mixing and increase in mass transfer resistance due to increased viscosity [89], and the worsening of the solid and liquid contact [88]. But, in industrial applications, it is desired to work at high solid/liquid ratios as much as possible due to economic factors.
3.2 Effects of time and H2SO4 concentration
The variation of the Cu extraction (%) at a constant solid/liquid ratio of 0.105, depending on the time and H2SO4 concentration, is given in Fig. 2.
Figure 2 shows that with the increase in time, the Cu extraction (%) increases and reaches its maximum values in the range of about 70–80 min at low H2SO4 concentration. At higher times, the Cu extraction (%) again decreased. In the range of 0.35–0.45 M H2SO4 concentration, the Cu extraction (%) did not change much depending on the time. At the higher H2SO4 concentration, the Cu extraction (%) values decreased rapidly to the minimum values in the range of approximately 70–80 min and reached the maximum values rapidly increasing again over 80 min. The highest Cu extraction (%) values were reached at the maximum values of the time and H2SO4 concentration. In other words, The Cu extraction (%) values increased due to the increase in the H2SO4 concentration in low and high times, whereas the Cu extraction (%) decreased as the H2SO4 concentration increased in the medium periods (in the range of 70–80 min).
Acid concentration in hydrometallurgical processes is one of the most significant variables to be controlled in terms of reaction kinetics and the economy [90]. It is known that increasing the acid concentration up to a certain value will increase leaching efficiency [91, 92]. At low H2SO4 concentration, with increasing the time up to 70 min, increasing Cu extraction (%) can be attributed to the increased interaction of the particles with H2SO4 concentration [86]. At low H2SO4 concentration, decreasing Cu extraction (%) due to the increase in time over 80 min can be attributed to the increase in pH value due to the dissolved oxygen in the solution [93, 94]. This decrease may also have been due to the precipitation of dissolved Cu. The fact that Cu extraction (%) does not change much in the concentration range of 0.35–0.45 M H2SO4 at all times can be explained by the reduction of possible contact of H2SO4 solution with the particle surface [55], due to the precipitation of ferric sulfate forms on the surfaces and pores of the particles [11, 95,96,97,98]. Similar evaluations can be said in the explanation of low Cu extraction (%) at high H2SO4 concentrations in the range of 70–80 min.
3.3 Effects of H2SO4 concentration and solid/liquid ratio
The change in the Cu extraction (%) depending on the H2SO4 concentration and solid/liquid rate at 65 min constant time is given in Fig. 3.
As seen in Fig. 3, both at low and high H2SO4 concentrations, Cu extraction (%) values tend to increase at all solid/liquid ratios studied. The low Cu extraction (%) values were obtained at medium H2SO4 concentration (between 0.3 and 0.5 M). Also, regardless of the H2SO4 concentrations studied, the Cu extraction (%) values increased with increasing solid/liquid ratio up to 0.1 and it decreased partially afterward. The reasons for the increase/decrease in Cu extraction (%) depending on both H2SO4 concentration and solid/liquid ratio are discussed in the previous paragraphs.
3.4 Optimization
The roasted chalcopyrite concentrate was leached at Experiment No.: A6 both with a Cu extraction (%) of 90.11% and the lowest grade such as 1.42% Cu in solid residue. Optimum time (X1; 10–120 min), solid/liquid ratio (X2; 0.01–0.20), and H2SO4 concentration (X3; 0.01–1.00 M) provided that selected parameters which are the most important independent variables in the chalcopyrite leaching, 115 min, 0.116 and 0.71 M, respectively, were determined, and the highest Cu extraction (%) value was calculated as 92.45%.
4 Conclusion
In this study, the suitability of Cu extraction (%) by using leaching after the roasting method from chalcopyrite concentrate was identified by using the Box–Wilson statistical experimental design method. The Box–Wilson statistical experimental design method was found to be viable for modeling the effects of significant parameters on the Cu extraction (%) of roasted chalcopyrite concentrates. Response function predictions based on regression analysis were well in line with the experimental results.
The effects of time, solid/liquid ratio and H2SO4 concentration on the Cu extraction (%), which are among the most important parameters effective in leaching of roasted chalcopyrite concentrate, were examined and the optimum parameters were determined as 115 min, 0.116 and 0.71 M, respectively, and the highest Cu extraction (%) value was calculated as 92.45%. Copper in the roasted chalcopyrite concentrate was leached with a 90.11% Cu extraction, and the copper grade in the solid residue was the lowest value as 1.42% Cu. It is seen that higher Cu extraction (%) values can be achieved with a study to be performed in the optimum parameters.
It may also be useful to examine parameters such as mixing speed, temperature and type of solvent that are effective in leaching. In addition, it will be useful to examine the behavior of some impurities such as Fe both in the leaching and in extraction from solution processes.
References
Biswas AK, Davenport WG (1974) Extractive metallurgy of copper, 2nd edn. Pergaman Press, New York, pp 64–69
Thorsen G (1980) Topics in non-ferrous extractive metallurgy. In: Burkin AR (ed) Extractive metallurgy of copper. Blackwell, Oxford, pp 1–41
Naguman PN (2008) The chemistry and kinetics of oxidized copper sulfiding by sodium thiosulfate. Russ J Non Ferrous Met 49:433–437
Salavati-Niasari M, Davar F (2009) Synthesis of copper and copper(I) oxide nanoparticles by thermal decomposition of a new precursor. Mater Lett 63:441–443
Tsogtkhankhai D, Mamyachenkov SV, Anisimova OS, Naboichenko SS (2011) Thermodynamics of reactions during nitric acid leaching of minerals of a copper concentrate. Russ J Non Ferrous Met 52:135–139
Akçil A (2002) A preliminary research of acid pressure leaching of pyritic copper ore in Küre copper mine Turkey. Miner Eng 15(12):1193–1197
Bingöl D, Canbazoğlu M (2004) Dissolution kinetics of malachite in sulphuric acid. Hydrometallurgy 72:159–165
Cao ZF, Zong H, Liu GY, Zhao SJ (2009) Techniques of copper recovery from Mexican copper oxide ore. Min Sci Technol 19:45–48
Habashi F (1978) Chalcopyrite. Its chemistry and metallurgy. McGraw-Hill, New York
Padilla R, Vega D, Ruiz MC (2007) Pressure leaching of sulfidized chalcopyrite in sulfuric acid-oxygen media. Hydrometallurgy 86:80–88
Cordoba EM, Munoz JA, Blazquez ML, Gonzalez F, Ballester A (2008) Leaching of chalcopyrite with ferric ion. Part I: general aspects. Hydrometallurgy 93(3):81–87
Antonijevic MM, Jankovic ZD, Dimitrijevic MD (2004) Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid. Hydrometallurgy 71:329–334
Li Y, Qian G, LiJ GAR (2015) Kinetics and roles of solution and surface species of chalcopyrite dissolution at 650 mV. Geochim Cosmochim Acta 161:188–202
Nazari G, Dixon D, Dreisinger D (2011) Enhancing the kinetics of chalcopyrite leaching in the Galvanox™ process. Hydrometallurgy 105(3):251–258
Harmer SL, Thomas JE, Fornasiero D, Gerson AR (2006) The evolution of surface layers formed during chalcopyrite leaching. Geochim Cosmochim Acta 70:4392–4402
Sokic MD, Markovic B, Zivkovic D (2009) Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 95:273–279
Li Y, Kawashima N, Li J, Chandra AP, Gerson AR (2013) A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv Colloid Interface Sci 197–198:1–32
Qiu T, Nie G, Wang J, Cui L (2007) Kinetic process of oxidative leaching of chalcopyrite under low oxygen pressure and low temperature. Trans Nonferrous Met Soc China 17:418–422
Prasad S, Pandey BD (1998) Alternative processes for treatment of chalcopyrite—a review. Miner Eng 11(8):763–781
Liao Y, Zhou J, Huang F, Wang Y (2015) Leaching kinetics of calcification roasting calcinate from multimetallic sulfide copper concentrate containing high content of lead and iron. Sep Purif Technol 149:190–196
Hua Y, Cai C, Cui Y (2006) Microwave-enhanced roasting of copper sulfide concentrate in the presence of CaCO3. Sep Purif Technol 50:22–29
Watling HR (2013) Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate-chloride and sulfate-nitrate process options. Hydrometallurgy 140:163–180
Dreisinger D (2006) Copper leaching from primary sulfides: options for biological and chemical extraction of copper. Hydrometallurgy 83(1):10–20
Dutrizac JE (1989) Elemental sulphur formation during the ferric sulphate leaching of chalcopyrite. Can Metall Q 28(4):337–344
Marsden JO, Wilmot JC (2007) Medium-temperature pressure leaching of copper concentrates—part II: development of direct electrowinning and an acid-autogenous process. Miner Metall Process 24:205–217
Al-Harahsheh M, Kingman S, Al-Harahsheh A (2008) Ferric chloride leaching of chalcopyrite: synergetic effect of CuCl2. Hydrometallurgy 91:89–97
Olubambi PA, Potgieter JH (2009) Investigations on the mechanisms of sulfuric acid leaching of chalcopyrite in the presence of hydrogen peroxide. Miner Process Extr Metall Rev 30:327–345
Vracar RZ, Vuckovic N, Kamberovic Z (2003) Leaching of copper(I) sulphide by sulphuric acid solution with addition of sodium nitrate. Hydrometallurgy 70:143–151
Cordoba EM, Munoz JA, Blazquez ML, Gonzalez F, Ballester A (2008) Leaching of chalcopyrite with ferric ion. Part II: effect of redox potential. Hydrometallurgy 93(3):88–96
Prasad S, Pandey BD (1999) Thermoanalytical studies on copper-iron sulphides. J Therm Anal Calorim 58(3):625–637
İkiz D, Gülfen M, Aydın AO (2006) Dissolution kinetics of primary chalcopyrite ore in hypochlorite solution. Miner Eng 19(9):972–974
Hackl RP, Dreisinger DB, Peters E, King JA (1995) Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 39(1):25–48
Stott MB, Watling HR, Franzmann PD, Sutton D (2000) The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Miner Eng 13:1117–1127
Cordoba EM, Munoz JA, Blazquez ML, Gonzalez F, Ballester A (2009) Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 °C. Miner Eng 22(3):229–235
Viramontes-Gamboa G, Pena-Gomar MM, Dixon DG (2010) Electrochemical hysteresis and bistability in chalcopyrite passivation. Hydrometallurgy 105:140–147
Bingöl D, Canbazoğlu M, Aydoğan S (2005) Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 76:55–62
Ata ON, Çolak S, Ekinci Z, Çopur M (2001) Determination of the optimum conditions for leaching of malachite ore in H2SO4 solutions. Chem Eng Technol 24:409–413
Pradhan N, Nathsarma KC, Rao KS, Sukla LB, Mishra BK (2008) Heap bioleaching of chalcopyrite: a review. Miner Eng 21:355–365
Dutrizac JE (1992) The leaching of sulphide minerals in chloride media. Hydrometallurgy 29(1–3):1–45
Warren IH (1958) A study of the acid pressure leaching of chalcopyrite, chalcocite and covellite. Aust J Appl Sci 9:36–51
Thomas G, Ingraham TR, MacDonald RJC (1967) Kinetics of dissolution of synthetic digenite and chalcocite in aqueous acid ferric sulphate solution. Can Metall Q 106:281–291
Faris N, Ram R, Chen M, Tardio J, Pownceby MI, Jones LA, McMaster S, Webster NAS, Bhargava S (2017) The effect of thermal pre-treatment on the dissolution of chalcopyrite (CuFeS2) in sulfuric acid media. Hydrometallurgy 169:68–78
Elsherief AE (2002) The influence of cathodic reduction, Fe2+ and Cu2+ ions on the electrochemical dissolution of chalcopyrite in acidic solution. Miner Eng 15:215–223
Majuste D, Ciminelli VST, Osseo-Asare K, Dantas MSS, Magalhaes-Paniago R (2012) Electrochemical dissolution of chalcopyrite: detection of bornite by synchrotron small angle X-ray diffraction and its correlation with the hindered dissolution process. Hydrometallurgy 111–112:114–123
Debernardi G, Carlesi C (2011) Chemical-electrochemical approaches to the study passivation of chalcopyrite. Miner Process Extr Metall Rev 34:10–41
Ruiz MC, Montes KS, Padilla R (2011) Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy 109:37–42
Nazari G, Asselin E (2009) Morphology of chalcopyrite leaching in acidic ferric sulfate media. Hydrometallurgy 96(3):183–188
Mwase JM, Petersen J, Eksteen JJ (2012) A conceptual flowsheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate. Hydrometallurgy 111–112:129–135
Sullivan JD (1933) Chemical and physical features of copper leaching. Trans AIME 106:515–546
Wang X, Chen QY, Hu HP, Yin ZL, Xiao ZL (2009) Solubility prediction of malachite in aqueous ammoniacal ammonium chloride solutions at 25 °C. Hydrometallurgy 99:231–237
Sun XL, Chen BZ, Yang XY, Liu YY (2009) Technological conditions and kinetics of leaching copper from complex copper oxide ore. J Cent South Univ 16:936–941
Venkatachalam S (1991) Treatment of chalcopyrite concentrates by hydrometallurgical techniques. Miner Eng 4(7–11):1115–1126
Sanchez EC, Umetsu Y, Saito F (1996) Effect of iron on copper extraction by acid leaching of chalcopyrite concentrate. J Chem Eng Jpn 29(4):720–724
Peters E (1992) Hydrometallurgical process innovation. Hydrometallurgy 29:431–459
Antonijevic MM, Bogdanovic GD (2004) Investigation of the leaching of chalcopyritic ore in acidic solutions. Hydrometallurgy 73:245–246
Cerda CP, Taboada ME, Jamett NE, Ghorbani Y, Hernández PC (2018) Effect of pretreatment on leaching primary copper sulfide in acid-chloride media. Minerals 8(1):1–14
Wang G, Liu Y, Tong L, Jin Z, Chen G, Yang H (2019) Effect of temperature on leaching behavior of copper minerals with different occurrence states in complex copper oxide ores. Trans Nonferrous Met Soc China (English Ed) 29(10):2192–2201
Cháidez J, Parga J, Valenzuela J, Carrillo R, Almaguer I (2019) Leaching chalcopyrite concentrate with oxygen and sulfuric acid using a low-pressure reactor. Metals 9(189):1–19
Uzun E, Zengin M, Atılgan İ (2016) Improvement of selective copper extraction from a heat-treated chalcopyrite concentrate with atmospheric sulphuric-acid leaching. Mater Technol 50(3):395–401
Arce EM, Gonzalez I (2002) A comparative study of electrochemical behavior of chalcopyrite, chalcocite and bornite in sulfuric acid solution. Int J Miner Process 67:17–28
Li J, Kawashima N, Kaplun K, Absolon VJ, Gerson AR (2010) Chalcopyrite leaching: the rate controlling factors. Geochim Cosmochim Acta 74:2881–2893
O’Connor GM, Eksteen JJ (2020) A critical review of the passivation and semiconductor mechanisms of chalcopyrite leaching. Miner Eng 154:106401
Çopur M, Kızılca M, Kocakerim MM (2015) Determination of the optimum conditions for copper leaching from chalcopyrite concentrate ore using taguchi method. Chem Eng Commun 202:927–935
Sokic M, Ilic I, Zivkovic D, Vuckovic N (2008) Investigation of mechanism and kinetics of chalcopyrite concentrate oxidation process. Metalurgija 47:109–113
Bayer G, Wiedemann HG (1992) Thermal analysis of chalcopyrite roasting reactions. Thermochima Acta 198(2):303–312
Dunn JG (1997) The oxidation of sulphide minerals. Thermochim Acta 300(1–2):127–139
Sahyoun C, Kingman SW, Rowson NA (2003) The effect of heat treatment on chalcopyrite. Phys Sep Sci Eng 12(1):23–30
Wang XW, Peng J, Wang MY, Ye PH, Xiao Y (2011) The role of CaO in the extraction of Ni and Mo from carbonaceous shale by calcification roasting, sulphation roasting and water leaching. Int J Miner Process 100:130–135
Ma SF, Zhang GX (2007) Leaching process of vanadium from argillaceous vanadium ore using calcified roasting study on roasting technology. Chin J Rare Met 31:813–817
Grishina EP, Udalova AM, Rumyantsev EM (2002) Anodic oxidation of copper in concentrated solutions of sulfuric acid. Russ J Electrochem 38(9):1041–1044
Habbache N, Alane N, Djerad S, Tifouti L (2009) Leaching of copper oxide with different acid solutions. Chem Eng J 152(2–3):503–508
Wang Y, Lany S, Ghanbaja J, Fagot-Revurat Y, Chen YP, Soldera F, Horwat D, Mücklich F, Pierson JF (2016) Electronic structures of Cu2O, Cu4O3, and CuO: a joint experimental and theoretical study. Phys Rev B 94(24):245418
Sun G, Alexandrova AN, Sautet P (2020) Structural rearrangements of subnanometer Cu oxide clusters govern catalytic oxidation. ACS Catal 10(9):5309–5317
Türkmen Y, Kaya E (2009) Acidified ferric chloride leaching of a chalcopyrite concentrate. J Ore Dress 11(22):16–24
Gök Ö, Anderson CG (2013) dissolution of low-grade chalcopyrite concentrate in acidified nitrite electrolyte. Hydrometallurgy 134–135:40–46
Gök Ö, Anderson CG, Çiçekli G, Cöcen Eİ (2013) leaching kinetics of copper from chalcopyrite concentrate in nitrous-sulfuric acid. Physicochem Probl Miner Proc 50(1):399–413
Türkmen Y, Kaya E (2010) Leaching of chalcopyrite concentrate in sulphuric acid with the aid of mechanical activation and microwave energy. Asian J Chem 22(10):8107–8116
Akçil A, Ciftçi H, Deveci H (2007) Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner Eng 20:310–318
Sargsyan LY, Hovhannisyan AM (2010) Activated sulfating roasting of the chalcopyrite concentrate for sulfuric acid leaching. Russ J Non Ferrous Met 51(5):386–388
Gülfen M, Aydın AO (2010) Dissolution of copper from a primary chalcopyrite ore calcined with and without Fe2O3 in sulphuric acid solution. Indian J Chem Technol 17:145–149
Gülfen M, Aydın AO (2008) Dissolution kinetics of calcined chalcopyrite ore in sulphuric acid solution. Indian J Chem Technol 15:180–185
Nyamjargal L, Batdemberel G, Burmaa G, Burmaa D (2018) Effect of roasting temperature for copper leaching of sulfide concentrate by combined methods. Open J Appl Sci 8:545–553
Bahar N (2015) Recovery of copper from chalcopyrite concentrate by sulfating roasting-acid leaching method. Int J Pure Appl Sci 1:72–78 (in Turkish)
Davies OL (1956) The design and analysis of industrial experiments. Hafner Publishing Co., New York
Crozier RD (1992) Flotation. Pergamon Press, Oxford
Shabani MA, Irannajad M, Azadmehr AR (2012) Investigation on leaching of malachite by citric acid. Int J Miner Metall Mater 19:782–786
Künkül A, Gülezgin A, Demirkıran N (2013) Investigation of the use of ammonium acetate as an alternative lixiviant in the leaching of malachite ore. Chem Ind Chem Eng Q 19(1):25–35
Tanaydın MK, Demirkıran N (2017) Malahit Cevherinin Perklorik Asit Çözeltilerindeki Çözünürlüğünün İncelenmesi. Çukurova Univ J Fac Eng Archit 32(3):175–185 (in Turkish)
Kim CJ, Yoon HS, Chung KW, Lee JY, Kim SD, Shin SM, Lee SJ, Joe AR, Lee SI, Yoon SJ, Kim SH (2014) Leaching kinetics of lanthanum in sulfuric acid from rare earth element (REE) slag. Hydrometallurgy 146:133–137
Dreisinger D, Abed N (2002) A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: kinetic analysis. Hydrometallurgy 66:37–57
Aydogan S, Ucar G, Canbazoğlu M (2006) Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution. Hydrometallurgy 81:45–51
Adebayo A, Ipinmoroti K, Ajayi O (2003) Dissolution kinetics of chalcopyrite with hydrogen peroxide in sulphuric acid medium. Chem Biochem Eng Q 17:213–218
Joe S, Chida T, Sakoda M, Nakamura H, Tamura M, Sato N (2009) Effect of sulfuric acid concentration on chalcopyrite concentrate chemical leaching. Adv Mater Res 71–73:353–356
Vilcaez J, Yamada R, Inoue C (2009) Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures. Hydrometallurgy 96:62–71
Lu ZY, Jeffrey MI, Lawson F (2000) The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 56:189–202
Lu ZY, Jeffrey MI, Lawson F (2000) An electrochemical study of the effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 56:145–155
Dutrizac JE, MacDonald RJC, Ingraham TR (1969) The kinetics of dissolution of synthetic chalcopyrite in aqueous acidic ferric sulfate solutions. Trans Metall Soc AIME 245:955–959
Pinches A, Al-Jaid FO, Williams DJA (1976) Leaching of chalcopyrite concentrates with Thiobacillus ferrooxidans in batch culture. Hydrometallurgy 2:87–103
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sönmez, İ., Şahbudak, K., Kartal, L. et al. Optimization of sulfuric acid leaching of roasted chalcopyrite concentrate with Box–Wilson experimental design. SN Appl. Sci. 2, 1557 (2020). https://doi.org/10.1007/s42452-020-03341-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03341-6