Abstract
Until recently, engineers either ignored or neglected the role of microorganisms in geotechnical engineering. The microbially induced calcite precipitation (MICP) research technique is an innovative and relatively green technology which consists in a biological process through which microorganisms react with minerals (calcium source and cementation reagent) to produce calcite (CaCO3) as a byproduct that modifies and improves the engineering properties of soil. Laboratory and field results obtained from various studies are promising and viable, suggesting potential for various engineering applications. This research technique has also been considered to be useful in many engineering applications such as improvement of construction materials, cementation of porous media, improvement in strength and stiffness of engineering soils, hydraulic control of engineering facilities (waste containment), liquefaction, and erosion mitigation. In this review, the various methods of production of calcium carbonates (calcite), the role played by bacteria, various state-of-the-art procedures that have evolved in this research area, as well as ongoing studies are reported. Furthermore, the use of the MICP technique in remediation of contaminants and other environmental concerns is also presented. The advantages and challenges (in form of undesired byproducts) of this research technique are highlighted herein.
Similar content being viewed by others
1 Introduction
A number of conventional approaches used in the treatment of soil for engineering use are known to engineers. Over the last century, various methods of ground-improvement techniques for improving soil by using chemical solution/grout have been established and are widely used in geotechnical engineering applications. Most of these methods of ground improvement not only end up contaminating the ground and environment but are also expensive [1]; this has become a source of concern to engineers and researchers. The demand for novel and innovative techniques for ground improvement has compelled researchers to look for new ways of satisfying society’s demand for land to meet infrastructural needs, while considering environmental concerns. Over the years, engineers and researchers have either neglected the role of microbial activities within soils or have not given it adequate attention [2]. Hence, research into innovative and alternative approaches to ground improvement has focused on the use of biological processes. These techniques combine the usage of microorganisms, cementation reagents, and biological methods, which naturally exist in soils, to improve their engineering properties [3]. This approach results in minimal release of carbon dioxide into the environment, making it ecofriendly. These methods have also been found to be adaptable to project conditions as well as having an increased certainty of execution [4]. Major factors to consider when employing this technique of soil improvement include the selection and proof of appropriate microorganisms for diverse applications, surroundings, cost effectiveness, the modification of maximum microbial activity in situ, and the biosafety of the application [5].
Biogeotechnology is a division of geotechnical engineering that applies biological processes to solve geotechnical difficulties. In other words, it is known as a biomediated soil enhancement method, generally being related to the chemical reaction system achieved and measured within soil by means of biological action, resulting in calcite precipitate as its byproduct, which modifies the engineering properties of the soil [6]. As reported [7], this research trend started in Australia in 2001, when it was reported in a Dutch newspaper that bacteria were used to reinforce sand and restore a monument. At first, microbial soil improvement was seen as difficult/impossible for civil engineers. Nevertheless, it became a serious option after an Australian research group [8] converted a bag of sand into calcareous sandstone.
There are several engineering applications of this research technique. However, currently, much attention is given to bioclogging and biocementation of soils. Bioclogging is aimed at reducing the permeability of soil and porous rocks through microbial action or products, while biocementation enhances the strength and stiffness properties of soil and rocks through microbial activity or products. These applications represent a branch of geotechnical engineering that provides an environmentally friendly treatment process by utilizing a low-viscosity fluid that can penetrate into deep soil stratum, thereby modifying the engineering properties of the soil by increasing its stiffness and strength, and diminishing its permeability [5, 9, 10]. This technique has low cost and requires minimum extra energy, as well as reducing the discharge of carbon dioxide underground and thereby decreasing greenhouse gases.
Microbially induced calcite precipitation (MICP) is an emerging area of research that has received increasing attention from researchers as a sustainable means of soil improvement [11, 12]. Mitchell and Santamarina [13] pioneered a study on biological considerations in geotechnical engineering that was aimed at the definition, assessment, and illustration of the significance of a few biological features and methods in soils and rocks. Also, the study focused on the stimulation of interest in the development of better understanding of the roles of biological processes and likely practicality in progressing knowledge and practice of geotechnical engineering. Literature has reported tremendous interest and attention from researchers, covering microbiology/biology, geotechnical engineering, some aspects of chemistry, and other related areas of study for improving the engineering properties of soil. As a result, the significance of multidisciplinary research, which is not restricted to geotechnical engineering, has been pointed out in the last decade [5, 9, 14,15,16,17,18,19,20,21,22,23,24,25].
Dejong’s research group [26] considered soil as a living environment, suggesting the prospect for original and workable solutions to geotechnical problems. Biogeochemically based soil improvement technologies will not replace in totality standard ground enhancement methods; nevertheless, the method is favorable because of its potential environmental friendliness. The aim of this review is to provide up-to-date information on the state of the art and most recent developments linked to the use of microorganisms in geotechnical engineering applications and to summarize existing or potential applications of this research technique, including its upscaling and field applications as well as undesired byproducts generated by the MICP technique.
2 Microbial activities in soil
Microbial activities can change the appearance of soil and increase its ability to withstand applied forces/loads by precipitating calcite, which binds soil particles [27]. According to Reichle [28], microbes constitute between 70% and 85% of the living component within soil systems, but a good understanding of their metabolic rate is essential to forecast correctly how microorganisms will act under different conditions [29]. The number of organisms in a single kilogram of soil at the surface is between 109 and 1012 [13, 30]. It was reported by Dejong and his research team [31] that a gram of soil could be home to 1012 bacteria, and over 106 bacteria could be present in a single gram of poorly graded soil. Yargicoglu and Reddy [32] also reported approximately 109 cells within a gram of soil. Research has shown that microorganisms, especially bacteria, are linked to the formation of carbonate minerals, as they also play an important role in carbon cycling [11].
Bacteria can differ in shape; they can be rod like, almost round or spiral, and the range of their cell thickness is between 0.5 and 3 μm [26, 30, 31, 33]. Their thickness can be reduced under stress settings to around 0.2 μm; they reproduce by fission naturally. Bacteria can withstand unfavorable conditions, and at low to elevated acidity or salinity some are spore-forming organisms, allowing them to withstand/survive in harsh conditions such as high pressures of several hundred bar and equally at temperatures from below the freezing to above the boiling point of water [34].
Microorganisms, in general, can reproduce very rapidly by fission, and their growth rate is exponential. The generation (reproduction) time for bacteria is 10 min, but 1 h is typically used; For example, starting with bacteria with a reproduction time of 1 h under an ideal condition, they could multiply to 224. With this high reproduction rate, microbial activity can be anticipated everywhere. In soil pore fluid, bacteria with size of 1 μm could reach approximately 108 bacteria/ml [13]. The presence of microorganisms in the soil may not cause any harm to the soil environment, as the majority are native species of the soil. According to Kucharski [8], the microorganisms used in the MICP technique using ureolysis (organisms that react to urea) can be from any of the genera Bacillus, Sporosarcina, Sporoloactobacillus, Clostridium, and Desulfotomaculum. Examples include Leptothrix discophora, Sporosarcina pasteurii (S. pasteurii), Bacillus coagulans, Bacillus pumilus, Bacillus megaterium, Bacillus subtilis, Bacillus licheniformis, Bacillus cereus, etc., which can be either Gram positive or negative.
3 Terminologies used in microbially induced calcite precipitation
3.1 Biomineralization
Biomineralization is the chemical change of the immediate surroundings by microbial action, resulting in the precipitation of calcite and associated minerals [3, 35,36,37,38]. Calcite crystals precipitated through biomineralization are mainly inorganic minerals. They can also include trace essential organic compounds, which can control the biomineralization process [39, 40].
The biological process that leads to the synthesis of minerals is called biomineralization [41]. The resulting products are composite in nature, being made up of both mineral and organic constituents. There are three mechanisms involved in the formation of biominerals: (1) Biologically controlled mineralization involves cellular actions that direct the development of minerals [38, 42]; (2) Biologically influenced mineralization is the process by which passive mineral precipitation is initiated by the presence of cell surface organic substances, e.g., extracellular polymeric substances, linked to biofilms [38, 40]; (3) The biologically induced mineralization process, in which precipitation occurs as an output of the interface between the environmental and biological activity; this depends substantially on the environmental conditions in the formation of the minerals [43]. The different mechanisms of biocalcification, i.e., those mediated through nitrogen cycles, are most important in soils and geological deposits [44]. It has been reported [45] that native bacteria could be used to induce calcite precipitation in an adequate measure to alter soil engineering properties, which can also be used to remedy various geologic hazards. Furthermore, the combination of the three mechanisms above is frequently operative in biomineralization processes [46].
3.2 Bioclogging
The aim of this process is to decrease the ability of soil and permeable rocks to allow the passage of fluid through them due to microbial activity or products. Bioclogging can therefore be used to close a leaky construction pit, landfill, or dike. One of the resulting of the bioclogging process is microbial production of water-insoluble polysaccharides in situ [5].
3.3 Biocementation
During MICP processes, carbonate ions are produced, and in the presence of excess dissolved calcium ions, calcite crystals are precipitated. These crystals form links between existing grains of sand, thereby preventing movement of the soil grains, which ultimately increases the strength and stiffness of the soil; this process is called biocementation [47]. It is a process by which microorganisms are used to increase the strength of soil through the production of soil particle binding materials, due to the addition of bacteria and cementitious mixtures in the soil. The soil cementitious materials are typically carbonates, silicates, phosphates, sulphides, and hydroxides [5].
Calcite is an attractive component to be studied in biocementation, because its formation is commonly found naturally [33]. The aim of biocementation is to improve the strength and stiffness properties of soil and rocks through microbial activity. Cementation mechanisms that cover or link separate soil particles could be naturally occurring cementation or microbially induced cementation; these progressively reduce the pore space in the soil fabric and hence decrease the permeability of the soil [9]. In addition, the formation of calcite cement is enhanced by the increase in pH of the immediate environment due to microbial metabolic activity.
3.4 Bioremediation
The increased rate of environmental degeneration presently experienced all over the world as a result of industrial activities, most of which result from the exploration of fossil fuels on all the continents of the world, has increased the threat not only to the ecosystem but also to the health of all creatures, including humans [48]. Bioremediation is a process that uses microbial metabolism in the presence of optimal environmental conditions and adequate nutrients to destroy (biodegrade) or transform (biotransform) contaminants, not only petroleum hydrocarbons but also other metals metalloids [49]. Bioremediation is a technique employed to immobilize contaminants in soil using microbes. The two basic strategies are biostimulation and bioaugmentation. An overview of the performance of MICP to decrease the environmental bioavailability of toxic metals and metalloids through the formation of metallic carbonates (calcites) has been documented in literature [49]. Torres-Aravena [50] summarized results from seven different studies using MICP applications and reported a removal efficiency of heavy metals of between 89.5% and 100% from waste water for 8 out of the 12 different species of organisms employed in their studies.
3.5 Biostimulation
Biostimulation includes an alteration of the environment to stimulate bacteria existing in the soil that are capable of bioremediation [48]. This can be achieved by the introduction of several procedures to regulate nutrients and electron acceptors such as carbon, nitrogen, phosphorus, or oxygen (e.g., in the form of molasses), which are adequate to limit microbial activities [51,52,53].
Biostimulation requires modification of a contaminated soil to provide a natural microbial population with a favorable environment that will allow them to destroy the target contaminant [54, 55]. Biostimulation is mostly preferred due to its stimulation and growth of natural microbes which are already used to the subsurface environment [33]. This also comes with some challenges, among which are obtaining uniform treatment throughout the location and accepting the increased time associated with stimulation and growth as well [20]. Furthermore, because urease-secreting bacteria mostly originate from the subsurface, biostimulation by MICP could be a sustainable method for in situ stabilization of definite elements or in situ improvement of the load-bearing capacity of soil [32]. Mahanty [44] suggested that biostimulation appears to be an essential pretreatment for if not a substitute to bioaugmentation in an engineered soil biocalcification process system.
3.6 Bioaugmentation
Bioaugmentation is a biological method in MICP in which foreign bacteria are introduced into the soil to precipitate minerals or biofilms within the soil environment, with or without growth media [45, 56]. In most studies conducted to date, biostimulation has been the primary strategy used, while bioaugmentation has been built on bioremediation techniques developed over 30 years ago [26].
Studies by LaRock and Donovan [57] showed that over 99% of microbial microbes cultured and grown in the laboratory and then reintroduced back into the same soil from which they were cultured failed to survive in another environment that was different from where they were originally cultured and dominate the community. Several researchers [26, 58] have reported bioaugmentation (introduction of foreign microbes into an environment) to be considered less favorable than the biostimulation method (introduction of microbes into an environment in which they exist), in that it sometimes requires the introduction of foreign microbes. The challenges associated with obtaining a uniform application due to filtration of microbes in the subsurface, especially for soils having smaller pore spaces, also constitute a huge task. Another difficulty experienced with this method is that imported bacteria added into natural soils are likely to degenerate quickly in number due to predation and competition as well as stress from abiotic factors [58, 59], although some studies have shown an enhancement in bacterial delivery when surfactant is used in microbially induced calcite precipitation [31, 45, 60]. These challenges can be overcome by manipulating the bacteria that already exist in the soil (indigenous microbes).
4 Mechanisms of microbially induced calcite precipitation
In microbially induced calcite precipitation (MICP), calcite is the desired product responsible for improving the soil properties. MICP can take place whenever metabolic processes of microorganisms result in alteration of the surrounding aqueous phase, which results in the formation of calcite [38]. One of the ways this may be facilitated is through the attachment of the microorganisms in the porous subsurface environment. MICP occurs as a byproduct of collective metabolic activities by microbes through any of the following processes: urea hydrolysis, photosynthesis, sulfate reduction, denitrification (nitrate reduction), ammonification, or methane oxidation, which result in an increase in the saturation of calcium carbonate [3, 6, 14, 38, 42, 61,62,63,64,65]. Among the various processes through which calcite is formed as stated above, studies reported by Zhu and Dittrich [66] have shown that urea hydrolysis is the most well-developed technology in use. Mujah [67] also reported that ureolysis is most preferred by researchers because it is straightforward for the precipitation of calcite (CaCO3) and can also achieve up to 90% chemical conversion efficiency of calcite in less than 24 h, followed by photosynthesis. Zhu and Dittrich [66] also reported sulfate reduction as another MICP process that is widely studied, although its engineering advantage is commonly given less priority.
The different reactions and byproducts generated in the formation of calcite using the various MICP processes stated above are as follows:
-
1.
Urea hydrolysis (ureolysis) using ureolytic bacteria [68]; in the presence of urease-positive microorganisms, this reaction occurs: CO(NH2)2 + 2H2O + Ca2+ + Cell → 2NH +4 + Cell-CaCO3 with NH +4 as the byproduct.
-
2.
Photosynthesis using cyanobacteria algae [69]; 2HCO −3 + Ca2+ → CH2O + CaCO3 + O2 with O2 as the byproduct.
-
3.
Sulfate reduction using sulfate-reducing bacteria [70]; SO 2−4 + 2[CH2O] + OH− + Ca2+ → CaCO3 + CO2 + 2H2O + HS− with CO2 and HS− as the byproducts.
-
4.
Denitrification (nitrate reduction) using nitrate-reducing bacteria [65]: (1) CH2COO− + 2.6H+ + 1.6NO −3 → 2CO2 + 0.8N2 + 2.8H2O when the reaction is complete, with CO2 + N as byproducts; (2) Ca2+ + CO2 (aq) + 2OH− → CaCO3(s) + H2O when the reaction is incomplete, with NO and N2O as byproducts.
-
5.
Ammonification using myxobacteria [71]; HCO−+ H2O → (CH2O) + O2 + OH−
-
(1)
HCO −3 + OH− → CO 2−3 + H2O
-
(2)
Ca2+ + CO 2−3 → CaCO3
-
(3)
with NH3 as the byproduct.
-
(1)
-
6.
Methane oxidation using methanogens [64]; anaerobic oxidation: CH4 + SO 2−4 + Ca2+ → CaCO3 + H2S + H2O
Aerobic oxidation: CH4 + 2O2 → CO2 + 2H2O, with H2S being the byproducts for both the anaerobic and aerobic reactions. A detailed overview of the various mechanisms involved in MICP using different organisms is presented in Ref. [72].
Calcite is one of the most common and well-known minerals on Earth, comprising about 4% of the crust by weight [73,74,75]. MICP is currently being studied because of its various potential applications, such as soil improvement [5, 75, 76], sequestration of contaminants [77], mitigation of seismic liquefaction [9], and remediation of cracks in concrete and stone monuments [78,79,80,81,82,83,84,85,86], among others. Some of these applications include in situ improvement of soils [26, 30, 31, 33, 87,88,89]. This requires better understanding of the interface between microbial ecology and aqueous geochemistry. Urease activity is exhibited by various species of microorganisms [40], and the ability of urease to induce carbonate precipitation has been studied by several researchers [25, 58, 90,91,92,93,94].
Soils improved through urease-induced mineralization or cementation are environmentally friendly, as they can substitute ordinary Portland cement, which has been reported to produce large quantities of carbon dioxide through its production processes [22]. Chou [16] further reported that the hydrolysis of urea was promoted in completely stirred tank reactor (CSTR) and hydraulically completely mixed biofilm reactor (CMBR) systems containing loose or dense sand specimens that were inoculated with growing bacterial cells (S. pasteurii). They also reported a general increase in the friction angle of all the treated soil specimens when compared with untreated specimens. Xu [95] reported that the frequency of precipitation for calcium lactate was more than twice that for calcium nitrate, signifying that an organic source of calcium lactate may be more useful for cell activity, which is directly linked to the urease activity and calcite deposition. An example of a typical reaction that occurs during the MICP process (ureolysis) is shown in Fig. 1.

(After [6], with permission from Elsevier)
Overview of biomediated calcite precipitation using ureolysis.
4.1 Factors that affect the microbially induced calcite precipitation technique
The biocalcification activity in soils is not always optimum but can differ to a great extent depending on physiochemical properties such as the temperature, the nutrient composition of the microenvironment, and the type, source, and activities of the organisms [44]. Several studies [43, 96,97,98] have also shown that there are mainly four factors that affect microbially induced calcite precipitation techniques: the concentration of the cementation materials (calcium ion, dissolved organic carbon), geometric compatibility, pH, temperature, and availability of nucleation sites. In addition to the above factors, there are other environmental factors such as concentration of bacteria, soil type, particle size of the soil sample, and viscosity of the bacterial solution that affect the technique, governing the performance of calcite precipitation [92, 99,100,101].
4.1.1 Concentration of cementation reagents
It is well documented in literature that a high concentration of cementation reagent (0.5–1.0 M) affects the precipitation technique by generating a significant amount of calcite. However, the formation of calcite precipitated at a lower concentration (0.05–0.25 M) is more effective [101, 102]. The results are consistent with the findings reported in Refs. [9, 103].
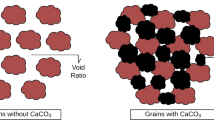
4.1.2 Geometric compatibility
Another factor that affects the MICP technique is the geometric compatibility of urease-producing microorganisms with the pore space of the soil used; that is, the largest and smallest soil particle size should be able to allow the free mobility of the microbes within the setup, as shown in Fig. 2.

(After [6], with permission from Elsevier)
Comparison of typical sizes of soil particles and bacteria, geometric limitations, and approximate limits of various treatment methods.
4.1.3 pH
A medium that is favorable to carbonation (i.e., the process that leads to formation of calcite) is normally alkaline; the optimal pH for microbial ureases is mostly near neutral. Stocks-Fischer [35] reported an optimum pH of 8 when using S. pasteurii, while other researchers who used the same S. pasteurii reported different optimal pH values; e.g., Dupraz [104] reported a pH value of 8.7–9.5, a pH value of 9.1 was reported in Ref. [105], and a pH of 9.5 has also been reported [106]. During the hydrolysis of urea, the pH of the environment generally increases throughout the microbially induced calcite precipitation process, and this acts to buffer the overall increase in pH value.
4.1.4 Temperature
Temperature has also been found to significantly influence the urease activity. At temperatures below 5 °C, urease activity is negligible, whereas in the range of 25–60 °C, Whiffin [107] reported an increase in urease activity in S. pasteurii that is directly proportional to temperature. However, an optimum was achieved at 70 °C, reducing thereafter to almost half the optimum value at 80 °C. In a similar study [108], the urease activities of B. megaterium were similar at different temperature conditions; however, at high temperature, B. megaterium exhibited lower enzyme activity, while at low temperature, it surpassed that of S. pasteurii. That study also reported that adding urea to the medium at the time of inoculation of the organisms could effectively overcome the low calcium precipitation associated with low urease activities at low temperature and enable subsequent low-temperature engineering applications.
4.1.5 Availability of nucleation sites (bacterial suspension density)
Stocks-Fischer [35] reported that the bacterial suspension density acts as nucleation sites for calcite precipitation processes, and this was confirmed [95]. The increase in the concentration of microbes has been reported [109] to be proportional to the amount of precipitated CaCO3, as long as it does not exceed the pore volume. Also, higher bacterial suspension density delivered into the soil leads to an increase in the MICP process [110].
The proportion of urea hydrolysis is directly proportional to the bacterial suspension density, provided there is enough calcium source for the reaction [30, 33, 40]. Martinez [20] demonstrated that the most important factor for achieving even calcite precipitation is the circulation of microbes. Burbank [58] also reported that native bacteria can be used to induce calcite precipitation and to substantially increase the liquefaction resistance of sands. The authors further demonstrated that the use of native bacteria at low flow rates results in even delivery of calcite precipitation points at or lower than 4% by weight of the soil sample. In summary [111], it is suggested that the bacterial cell density and urea and calcium ion concentrations are important factors for effective calcite precipitation via MICP processes.
Neupane [112] reported the enzymatic calcite precipitation performance, which was evaluated for in situ applications on a larger scale; a visible precipitation ratio was observed even with an insignificant quantity of enzyme. Furthermore, Soon [101] observed that the greatest enhancements in unconfined compressive strength and decrease in hydraulic conductivity were achieved at 100% and 90% relative densities, respectively, in which different suspension densities of S. pasteurii and cementation reagents were used. Other controlling parameters considered for the improvement were the flow rate of the cementation solution and the treatment period.
4.2 MICP research: laboratory and field studies
Several laboratory methods (Fig. 3), including studies of the injection method, injection volume, influence of the calcium source, concentration of cementation reagent, and factors that affect the MICP process, have been investigated with the aim of using the findings for various engineering applications. Commonly found soil bacteria (S. pasteurii, Bacillus coagulans, and Bacillus pumilus) were cultured from a tropical residual soil (lateritic soil) collected from a site in Abagana (latitude 6° 10′ 15″ N, longitude 6° 58′ 10″ E), Anambra State, which met the geometric compatibility requirements for this research technique, obtaining positive results [113,114,115].

(After: [7], with permission from ASCE)
Laboratory studies using MICP
Any novel and innovative research technique such as MICP will be better appreciated if the findings are geared toward solving real-life engineering problems. To achieve this aim, many studies have been conducted to upscale the MICP technique for field applications such as remediation of cracks in concrete, cementation of porous media, improvement in strength and stiffness, remediation of contaminants (heavy metals), etc. van Paassen [7] carried out the first full-scale application of the MICP technique, producing very promising findings (Figs. 3 and 4). The author was able to overcome the problem of borehole instability/collapse when horizontal directional drilling was adopted to treat a 1000 m3 volume depth that varied between 3 m and 20 m below the ground surface with 200 m3 of microbe and 300–600 m3 of cementation reagent, as shown in Fig. 5. Furthermore, the long-term performance/sustainability of MICP, the ecofriendliness of microbial activity in the soil, as well as the production cost of the test bacteria must be extensively clarified/studied in detail before field applications [116].

(After: [117], with permission from IOS Press)
Upscale studies of 100 m3 using MICP.

(After: [7], with permission from ASCE)
First field application of MICP.
4.3 MICP applications
MICP has recently been studied for a varied range of engineering uses [38]. Among these applications are amending or improving construction materials and remediation of cracks/self-healing in concrete [3, 72, 79,80,81,82,83,84,85,86, 102, 118,119,120,121,122], as well as cementation of porous media and improvements in strength and stiffness [6, 7, 26, 35, 103, 117, 121, 123]. It is also used in environmental remediation of heavy metals and radionuclides, including the potential to reduce subsurface leakage in the background of geologically isolated carbon dioxide [72, 104, 105, 124,125,126,127,128,129,130,131,132,133,134,135]. Recent studies [136] have reported MICP as a conservative method for restoring the lost strength in decayed limestone through biomineralization processes, for trial tests in both laboratory and field conditions.
The results of the MICP research technique of importance for engineering applications are the increase in the strength and stiffness (shear strength) of soil through biocementation and the reduction in the hydraulic conductivity through bioclogging of the soil pores/voids, which is a desired effect for waste-containment facilities, sealing of cracks in concrete, liquefaction mitigation, erosion control, and remediation of contaminants. Some of these engineering properties are discussed below:
4.3.1 Physical properties of soils
Since the discovery of MICP, little information has been reported in literature on the plasticity behavior of soil in MICP applications. It was reported earlier that fine-grain soils are not good candidates for MICP due to compatibility-related issues; however, Yasodian [137] reported an improvement in plasticity indices of seven different samples of residual soils (five clay soils, one bentonite, and one lateritic soil) after varying the treatment options in MICP. The liquid limit values of the clay soil ranged from 76% to 120% before treatment, which was reduced to the range of 55% to 97%. The plasticity index values ranged from 32% to 55% before treatment, which was reduced to the range of 5% to 23% after treatment. Greater reductions were recorded in the plasticity characteristics of the unsterilized compared with the sterilized specimens, suggesting that other microbial species may also be taking part in the MICP processes. There was a reduction in the clay content from 47% to 30% and an increase in the silt content from 29% to 48% after microbial treatment. There was a reduction in the plasticity index of the bentonite after treatment from 187% to 158%; this was found to further decrease to 141% after 15 days of curing. This could be a demonstration of the durability of the MICP process. There was a marginal increase in the liquid limit and plasticity index properties of the lateritic soil after treatment, despite the fact that it contained about 87.7% sand and silt, reported to be a compatibility requirement for effective MICP process. This finding was contradicted by Chittoori [138], who reported an increase in both the liquid limit and plasticity index of two clay soils and recorded a substantial reduction in the one-dimensional swell strain after bacterial treatment, with corresponding calcite content of 1.60% and 0.90%. Similarly, studies conducted by Osinubi [139] on lateritic soil after varying the ratio of bacteria to cementation reagent showed that an optimal improvement of plasticity index was achieved for specimens prepared with 75% S. pasteurii and 25% cementation reagent at S. pasteurii suspension density of 2.40 × 109 cells/ml, with a peak calcite content of 6.0%. This was due to the higher suspension density of S. pasteurii that were available to maximally hydrolyze the urea within the cementation reagent.
4.3.2 Shear strength characteristics
One of the targets of soil improvement for engineering use is shear strength gain. For biomediated improvement of soil, van Paassen [7, 14, 15, 117] demonstrated an increase in shear strength values. Similarly, Li [140] reported a considerable increase in unconfined compressive strength (UCS) when asparaginase enzyme and Bacillus megaterium were studied (980 kPa, 1002 kPa), but the sample failed as biogrout due to the lack of calcite precipitates in the absence of bacterial cells. They recommended the use of the enzyme (asparaginase) in the MICP technique rather than the urease bacteria (Bacillus megaterium), because it releases less ammonia to the environment (i.e., 40.6 U ml−1 versus 592 U ml−1 released by Bacillus megaterium). Furthermore, the asparaginase-based MICP process does not require urea, which emits secondary pollution. Yasuhara [141] reported UCS values ranging from 400 kPa to 1.6 MPa when sand was improved through MICP, while an increase in the UCS value of about 5% compared with the untreated specimen was also reported [22].
Gomez [142] reported UCS values ranging from 1.07 MPa to 5.34 MPa for average calcite contents of 6.0–13.2%. Putra [145] reported an increase in the UCS of a soil treated with MICP to a maximum value of 470 kPa. Also, an increase in the UCS from 150 to 198 kPa, compared with values in the range of 119–137 kPa for the control specimen, was reported [143]; meanwhile, Stabnikov [90], reported an increase in the UCS value from 765 kPa to 845 kPa. Furthermore, an increase in the strength of fiber-reinforced sand by means of the MICP technique was also reported [144]. An optimum 0.2–0.3% fiber content in the MICP-treated sand was established to be more than two times higher than the control, i.e., those that had no fiber added.
In a study to mitigate soil erosion [146], a maximum increase of 0.6 MPa in the UCS of a soil treated with MICP was obtained. Studies by Jijian [147] revealed an improvement in the UCS of a sand column of up to 1.91 MPa after employing a biogrouting method which effectively hardened black sands. To demonstrate the influence of treatment cycles in MICP [148], an increase in the UCS of up to 14 MPa after 32 treatment cycles using a percolation method with corresponding calcite content of 22% was reported. Similarly, an increase in the UCS value of lateritic soil when treated with Bacillus pumilus and S. pasteurii was also reported [115, 149]. They observed no gain in strength when specimens were cured in a humid environment; but a significant increase in UCS value was recorded when specimens were cured at room temperature (i.e., 24 ± 2 °C) for 2 days. The UCS value of the lateritic soil increased upon treatment with bacteria, from 66.74 kPa for the natural soil to 2345.15 kPa at 2.40 × 109 cells/ml suspension density using Bacillus pumilus, and from 120 kPa for the natural soil to 2232 kPa at 1.20 × 109 cells/ml suspension density using S. pasteurii.
4.3.3 Hydraulic conductivity
The hydraulic conductivity (permeability) of a soil is one of the most important parameters involved and used in the assessment of engineering facilities for dams and waste containment applications regarding contaminant migration into the subsurface [150]. Because of the significance and sensitivity of hydraulic conductivity in waste containment facilities, their values are not frequently rounded up but given to at least two significant figures [151]. A reduction in the value of the hydraulic conductivity by 60–70% for all the sand treated using the MICP technique was reported [141]. Furthermore, both the hydraulic conductivity and shear strength of biocemented soils were also reported [152], giving results that support MICP as a promising soil-improvement method.
Various researchers have reported the reduction in the permeability of soils treated with MICP. Soon [153] reported a reduction in the hydraulic conductivity from 1 × 10−7 to 2.6 × 10−8 m/s after treatment of residual soils. Also, a reduction in the hydraulic conductivity by as much as four orders of magnitude has been reported, depending on the effective size of the soil specimen (D10), average calcite content, and initial hydraulic conductivity [142]. Furthermore, a maximum reduction of 98.18% in the hydraulic conductivity of compacted lateritic soil treated at 2% water content with a 6.0 × 108 cells/ml suspension density of S. pasteurii was also reported [154].
Wiszniewski [155] reported a reduction in the hydraulic conductivity when two different samples of sandy soils were treated with 0.1–1.5% xanthan gum from the bacterial species Xanthomonas campestris; the reductions recorded were from 7.16 × 10−3 to 5.75 × 10−5 m/s and from 8.46 × 10−3 to 2.84 × 10−11 m/s for the two samples, respectively. Some species of the organisms used in MICP processes have the potential to form a biomass or biofilm: these substances have been reported to be linked with factors responsible for the reduction in the hydraulic conductivity, in addition to blockage of voids in the soil medium by calcite precipitate [152, 153, 156].
4.3.4 Waste containment applications and contaminant attenuation
After the discovery of MICP over a decade ago, the attention of researchers in geotechnical engineering has been focused on studies targeting either soil strength improvement or reduction of hydraulic conductivity; however, a search of recent literature reveals that studies on the application of MICP in waste containment applications have not only been reported but have also shown positive results. The following have been reported as the basic requirements needed for a material to be considered suitable for use as a liner in waste containment facilities: a maximum hydraulic conductivity value of 1 × 10−9 m/s to guarantee that the contaminant in the leachate from the waste can successfully be diminished and controlled to properly safeguard against pollution of groundwater/drinking water [157, 158], a minimum UCS of 200 kN/m2 to ensure stability against forces capable of destroying the material connection [159], and a maximum volumetric shrinkage of 4% [160]. In addition to these parameters, compatibility has recently been incorporated as a further parameter when evaluating materials for use in liner and waste containment facilities for excellent performance [161]. A material is said to be compatible if its interaction with the leachate does not result in compromise of, especially, the hydraulic properties [162]. One research group employed three different species of urease-positive microorganisms, i.e., Bacillus pumilus, Bacillus coagulans, and S. pasteurii, to evaluate tropical residual soil (lateritic soil) for use as a liner in waste containment facilities using the MICP approach [114, 115, 163,164,165,166,167,168,169,170,171]. Positive results have been reported for all the required parameters for evaluation of these materials, viz. UCS [114, 115, 163], hydraulic properties [113, 154], volumetric shrinkage [164,165,166], and compatibility [72, 167,168,169,170,171]. The results of these studies are not only viable but also promising, and more years of exciting research opportunities from laboratory studies to full implementation of these research findings at larger scale lie ahead.
4.3.5 Liquefaction mitigation and erosion control
Liquefaction and erosion mitigation is an emerging area of research for MICP application. Liquefaction may occur due to soil deformation triggered by rapid loading such as earthquakes and vibrations, and cyclic loading on saturated cohesionless soils in undrained conditions, under which the soil tends to act like a liquid, hence the shear strength and stiffness are extremely reduced [172]. The long-term potential of MICP for mitigating earthquake-induced soil liquefaction has recently been reported in literature, based on two stages: stage 1 occurs through a process known as microbially induced desaturation and precipitation (MIDP), which involves interparticle cementation, void filling, and particle roughening; this is followed by stage 2, which marks significant strength development and stiffness, dilatant behavior, and cyclic strength of the soil, thereby resulting in long-term liquefaction mitigation of the soil [173, 174]. It was also reported [175, 176] that the use of MICP treatment can completely alter the liquefaction failure mode from flow failure to cyclic mobility, and significantly vary the excess pore pressure generation response of initially loose specimens.
Erosion mitigation using MICP has also been reported [177,178,179,180]; while they differ in the methods adopted, most studies have reported the mass loss and penetration resistance. The suppressive ability of MICP for controlling wind erosion has recently been studied by subjecting MICP-treated soils to wind tunnel conditions at different intervals, after which the mass loss due to erosion was determined. The reported results [177] demonstrate the potential of MICP as an effective and environmentally friendly means of airborne fugitive dust control; a maximum of 3.5% and minimum of 1.6% mass loss were recorded, and these values were also found to decrease with the treatment period. Anderson [178] reported a reduction in the mass loss from 0.828 g/cm2 for untreated sand to virtually zero when the medium contained bacteria at 0.5 ml per 100 g of sand at wind speed of 32 km/h; this mass loss was found to increase at higher wind speed, i.e., 48 km/h and 64 km/h. Also upon subjecting similar samples to all the wind speeds, only those samples with a medium containing at least 2 ml per 100 g of sand exhibited virtually no mass loss. A reduction in the total erosion mass upon MICP treatment has also been reported, due to bacteria–cementation reagent-related substances, i.e., calcite precipitates, which are not only harmless but also effective and environmentally friendly [179]. Similarly, there was a reduction in mass loss due to erosion in the treated soil when compared with the natural soil [180]. These reductions were not directly proportional to the volume of bacterial suspension density used, being mainly due to the calcite precipitates formed [180].
4.3.6 Remediation of contaminants
Contaminants are mostly generated by industrial processes such as metal plating, tanning, battery production, paper production, and pesticide synthesis [50]. Over the years, several traditional methods such as physicochemical extraction, soil washing, stabilization, and excavation have been used for the remediation of contaminants; however, these methods have been reported to suffer from high costs associated with the energy requirements [181]. MICP processes have been used for remediation of contaminants, as reported in literature, with varying degrees of success [61, 127,128,129,130,131,132,133,134, 181,182,183]. Jain and Arnepalli [184] reported that bioremediation involves several chemical and biological approaches such as biomineralization, bioaccumulation, biosorption, bioimmobilization, phytoremediation, etc. They further stated that it is a cost-effective and environmentally friendly remediation technique, as it remediates contaminants by adsorption. Remediation using MICP through ureolytic processes is effective because the products (calcites) are not sensitive to redox potentials, thereby hindering release of the contaminants back into the environment [127, 128]. A microorganism can be used for remediation of contaminants if it possesses any of the following characteristics: the ability to utilize, transform, degrade, or grow in the presence of the contaminants, thus toxicity testing is essential, as reported in Ref. [139]. Note that some of these contaminants are essential for cell growth, although they may be toxic at high concentrations due to serious health-related issues.
5 Advantages of the MICP technique
Considering the economic aspects of this soil improvement technique, Ivanov and Chu [5] gave a raw material cost estimate for microbial grouting of US $0.5/m3 to US $9.0/m3, while the total cost of the MICP treatment (i.e., material, equipment, and installation) in saturated soils was reported to range from US $25/m3 to US $75/m3 [26]. However, similar studies [185] have shown that materials and costs can be reduced through more effective cementation, while Karol [186] reported that the cost of placement for chemical grouting can be a major part of the total cost.
Apart from the greenhouse effect associated with the production of cement, the cost of producing materials needed in biogrout is far cheaper when compared with the cost of cement production [187, 188]. The MICP technique is based on natural processes that require less energy; it can be carried out at ambient temperature, beneath existing structures without disturbing them, and can allow improvement over a large area. Studies [15, 20, 73, 149, 189] have also shown that MICP treatment of soil results in a substantial increase in its strength, stiffness, and dilative behavior, resulting in improved geotechnical properties of the soil in order to solve several engineering problems.
5.1 Challenges in the MICP technique
5.1.1 Limitations of microbes
Most MICP laboratory studies have been carried out under nutrient-rich conditions [130], while in practical applications, such as soil reinforcement, metal remediation, repair of cracks in concrete, etc., such conditions are quite rare. Furthermore, microbes may be exposed to unfavorable conditions such as high pH, high temperature and pressure, desiccation, evaporation, oxygen and nutrient deficiency, salt concentration, etc. in the field.
Field conditions limit the bacterial strains within the soil, which is a requirement for MICP to occur. Although several reported field studies were conducted at high pressure, most bacteria used in MICP have been cultured at ambient temperature and pressure [190]. However, it is difficult for bacteria cultured in the ambient temperature range of 20–37 °C to survive or maintain their microbial activities at a temperature of 120 °C [191].
5.1.2 Undesired byproducts
Soil improvement using MICP is a novel and innovative technique compared with the conventional methods that have been in use for environmental applications. Undesired byproducts (e.g., ammonium, ammonia, hydrogen sulfite, nitrite, nitrous oxide, and carbon dioxide) generated during the various MICP processes may pose environmental and health risks [64, 65, 68, 70, 71, 192]; For example, ammonium (the byproduct of ureolysis) is very costly to treat in groundwater. van Paassen [15] reported that, if an incomplete reaction occurs during denitrification (a method used in MICP), greenhouse gases such as nitrite and nitrous oxide will be produced. Alley [193] reported that nitrous oxide has an atmospheric lifespan of 114 years and is a dominant greenhouse gas, having a 300-fold greater potential for global warming effects compared with carbon dioxide.
Another undesired byproduct of the MICP process is the toxic and combustible H2S gas released by sulfate-reducing bacteria, which results in many environmental and health issues [194]. However, undesired byproducts can be mitigated by: (1) identifying other strategies or techniques to prevent their generation, (2) using the byproducts for other applications nearby, or (3) adopting treatments to eliminate the byproducts [66].
5.1.3 Nonuniform injection of microbes and cementation reagents
The presence of microorganisms, sufficient nutrients, and cementation reagents in a soil medium is required for the beneficial application of the MICIP technique [66]. Dejong [31] reported that both the nutrients and cementation reagents can rapidly be exhausted due to: (1) the flow rates through the soil medium being too fast for reaction to occur, (2) nutrients not being provided in sufficient quantity, or (3) nutrients being exhausted over time. To overcome some of these challenges, studies [4, 20, 24, 109, 195] have been conducted to determine or optimize the quantities of injection of the microbes, nutrients, and cementation reagents. Some of the reported findings of the studies are: (1) The stop-flow injection method is preferred over continuous and recirculation methods of injection, as it provides an even and more uniform distribution of calcite when compared with the other two methods, which either result in greater filling of voids near the injection points or flushing of some of the microbes, preventing their participation in the precipitation process; (2) A maximum of two-thirds of the pore volume should be used as the injection volume for the microbes, nutrients, and cementation reagents [24]: (3) It is recommended [24] that a maximum of one-third of the pore volume should be used for the injection of microbes, an approach that yielded positive results with tropical residual soils [113,114,115].
6 Conclusions
Microbially induced calcite precipitation (MICP) is a sustainable green technique for improving the engineering properties of soils. The six mechanisms of MICP reviewed herein (urea hydrolysis, photosynthesis, sulfate reduction, denitrification, ammonification, and methane oxidation) reveal that only photosynthesis yields a desirable byproduct, while the remaining mechanisms generate undesired byproducts that still represent challenges for this technique. There is a direct relationship between the UCS of treated soils and the bacterial suspension density in MICP processes. Also, there is an indirect relationship between the hydraulic conductivity (permeability) and bacterial suspension density, which is an important parameter when MICP treatment of soil is considered for waste containment facilities. For the MICP technique to be effectively used for the improvement of soil properties, geometric compatibility, nucleation (bacteria mostly exist within the soil environment), a temperature range of 25–70 °C, and a pH range of 8.0–9.5 must be satisfied. Based on this review, larger-scale use of MICP processes, targeting the improvement of the engineering properties for sandy and residual soils (expansive and lateritic soils) for various engineering applications, represents a research opportunity that could be explored in the near future.
References
Faurel S, Laloui L (2011) A bio-hydro-mechanical model for propagation of biogrout in soils. In: Proceedings of geo-frontiers 2011: advances in geotechnical engineering, Dallas TX, ASCE, Geotechnical Special Publication, vol 211, pp 4041–4048
Ibrahim S, Hacer BO, Recep C, Esra B (2015) Bacteria-induced cementation process in loose sand medium. Mar Georesour Geotechnol 33(5):403–407. https://doi.org/10.1080/1064119X.2014.909912
Castro-Alonso MJ, Montañez-Hernandez LE, Sanchez-Muñoz MA, Franco M, Rubi M, Narayanasamy R, Balagurusamy N (2019) Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front Mater 6:126. https://doi.org/10.3389/fmats.2019.00126
Barkouki TH, Martinez BC, Mortensen BM, Weathers TS, De Jong JD, Ginn TR, Spycher NF, Smith RW, Fujita Y (2011) Forward and inverse bio-geochemical modeling of microbially induced calcite precipitation in half-meter column experiments. Transp Porous Med 90:23–39. https://doi.org/10.1007/s11242-011-9804-z
Ivanov V, Chu J (2008) Application of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Biotechnol 7(2):139–153. https://doi.org/10.1007/s11157-007-9126-3
DeJong JT, Mortensen BM, Martinez BC, Nelson DC (2010) Bio-mediated soil improvement. Ecol Eng 36:197–210. https://doi.org/10.1016/j.ecoleng.2008.12.029
van Paassen LA (2011) Bio-mediated ground improvement: from laboratory experiment to pilot applications. In: Proceedings of geofrontiers in geotechnical engineering 2011: technical papers, ASCE, pp 4099–4108
Kucharski ES, Cord-Ruwisch R, Whiffin V, Al-Thawadi SMJ (2006) Microbial biocementation. World Patent 066326
Dejong JT, Fritzges MB, Nusslein K (2006) Microbial induced cementation to control sand response to undrain shear. J Geotech Geoenviron Eng 132(11):1381–1392. https://doi.org/10.1061/(ASCE)1090-0241(2006)132:11(1381)
Lin H, Suleiman MT, Helm J, Brown DG (2014) Measurement of bonding strength between glass beads treated by microbial-induced calcite precipitation (MICP). In: Proceedings of geo-congress 2014: technical papers, ASCE, Geotechnical Special Publication, vol 234, pp 1625–1634
Hamdan N, Kavazanjian J, Rittman BE, Karatas I (2011) Carbonate mineral precipitation for soil improvement through microbial denitrification. In: Proceedings of geo-frontiers 2011: advances in geotechnical engineering, Dallas TX, ASCE, Geotechnical Special Publication, vol 211, pp 3925–3934
Dawoud O, Chen CY, Soga K (2014) Microbial induced calcite precipitation for geotechnical and environmental applications. In: Proceedings of new frontiers in geotechnical engineering 2014: technical papers, ASCE, Geotechnical Special Publication, vol 234, pp 11–18
Mitchell JK, Santamarina JC (2005) Biological considerations in geotechnical engineering. J Geotech Geoenviron Eng 131(10):11222–11233. https://doi.org/10.1061/(ASCE)1090-0241(2005)131:10(1222)
van Paassen LA, Daza CM, Staal M, Sorokin DY, van der Zon W, van Loosdrecht MCM (2010) Potential soil reinforcement by biological denitrification. Ecol Eng 36:168–175. https://doi.org/10.1016/j.ecoleng.2009.03.026
van Paassen LA, Ghose R, Van der Linden TJM, Van der Star WRL, Van Loosdrecht MCM (2010) Quantifying biomediated ground improvement by ureolysis. Large-scale biogrout experiment. J Geotech Geoenviron Eng 136(12):1721–1728. https://doi.org/10.1061/(asce)gt.1943-5606.0000382
Chou C, Seagen EA, Aydilek AH, Lai M (2011) Biocalcification of sand through ureolysis. J Geotech Geoenviron Eng 137(12):1179–1189. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000532
Al Qabany A, Mortensen B, Martinez B, Soga K, Dejong J (2011) Microbial carbonate precipitation: correlation of S-wave velocity with calcite precipitation. In: Proceedings of geo frontiers in geotechnical engineering 2011: technical papers, ASCE, pp 3993–4001
Al Qabany A, Soga K, Santamarina C (2012) Factors affecting efficiency of microbially induced calcium carbonate precipitation. J Geotech Geoenviron Eng 138(8):992–1001. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000666
Mortenson BM, Dejong JT (2011) Strength and stiffness of MICP treated sand subjected to various stress paths. In: Proceedings of geo frontiers in geotechnical engineering 2011: technical papers, ASCE, pp 4012–4020
Martinez BC, Dejong JT, Ginn TR, Montoya BM, Barkouki TH, Hunt C, Tanyu B, Major D (2013) Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J Geotech Geoenviron Eng 139(4):587–598. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000787
Feng K, Montoya BM, Evans TM (2014) Numerical investigation of microbial induced cemented sand mechanical behaviour. In: Proceedings of geo-congress 2014: technical papers, ASCE, Geotechnical Special Publication, vol 234, pp 1644–1653
Park S, Choi S, Kim W, Lee J (2014) Effect of microbially induced calcite precipitation on strength of cemented sand. In: Proceedings of new frontiers in geotechnical engineering 2014: technical papers, ASCE, Geotechnical Special Publication, vol 234, pp 47–56
Park S, Choi S, Nam I (2014) Effect of plant-induced calcite precipitation on the strength of sand. J Mater Civ Eng 26(8):06014017. https://doi.org/10.1061/(asce)gt.1943-5533.0001029
Rowshanbakht K, Kamehchiyan M, Sajedi RH, Nikudel MR (2016) Effects of injected bacterial suspension volume and relative density on carbonate precipitation resulting from microbial treatment. Ecol Eng 89:49–55. https://doi.org/10.1016/j.ecoleng.2016.01.010
Feng K, Montoya BM (2016) Influence of confinement and cementation level on the behaviour of microbial-induced calcite precipitated sands under monotonic drained loading. J Geotech Geoenviron Eng. 142(1):04015057. https://doi.org/10.1061/(asce)gt.1943-5606.0001379
Dejong JT, Soga E, Kavazanjian S et al (2013) Biogeochemical processes and geotechnical applications: progress, opportunities, and challenges. Geotechnique 63(4):287–301. https://doi.org/10.1680/geot.SIP13.P.017
Sari YD (2015) Soil strength improvement by microbial cementation. Mar Georesour Geotechnol 33(6):567–571. https://doi.org/10.1080/1064119X.2014.953234
Reichle DE (1977) The role of soil invertebrates in nutrient cycling. Ecol Bull 25:145–156
Epelde L, Becerril JM, Hernández-Allica J, Barrutia O, Garbisu C (2008) Functional diversity as indicator of the recovery of soil health derived from Thlaspi caerulescens growth and metal phytoextraction. Appl Soil Ecol 39(3):299–310. https://doi.org/10.1016/j.apsoil.2008.01.005
Umar M, Kassim KA, Tiong K, Chiet P (2016) Biological process of soil improvement in civil engineering: a review. J Rock Mech Geotech Eng. https://doi.org/10.1016/j.jrmge.2016.02.004
Dejong JT, Proto C, Kuo M, Gomez M (2014) Bacteria, bio-films and invertebrates… the next generation of geotechnical engineers. In: Proceedings of geo-congress 2014: technical papers, ASCE, Geotechnical Special Publication, vol 234, pp 3959–3968
Yargicoglu EN, Reddy KR (2015) Review of biological tools and their applications in geoenvironmental engineering. Rev Environ Sci Biotechnol 14:161–194. https://doi.org/10.1007/s11157-014-9358-y
Soon N, Lee L, Khun T, Ling H (2012) An overview of the factors affecting microbial-induced calcite precipitation and its potential application in soil improvement. Int J Civ Environ Struct Constr Archit Eng 6(2):188–194
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2008) Brock biology of microorganisms, 12th edn. Benjamin Cummings, San Francisco
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of CaCo3. Soil Biol Biochem 31(11):1563–1571
Barkay T, Schaefer J (2001) Metal and radionuclide bioremediation: issues, considerations and potentials. Curr Opin Microbiol 4:318–323
Rodriguez-Navarro C, Jroundi F, Schiro M, Ruiz-Agudo E, González-Muñoz MT (2012) Influence of substrate mineralogy on bacterial mineralization of calcium carbonate: implications for stone conservation. Appl Environ Microbiol 78(11):4017–4029. https://doi.org/10.1128/aem.07044-11
Phillips AJ, Gerlach R, Lauchnor E, Mitchell AC, Cunningham AB, Spangler L (2013) Engineered applications of ureolytic biomineralization: a review. Biofouling 29(6):715–733. https://doi.org/10.1080/08927014.2013.796550
Yoshida N, Higashimura E, Saeki Y (2010) Catalytic biomineralization of fluorescent calcite by the thermophilic bacterium Geobacillus thermoglucosi-dasius. Appl Environ Microbiol 76:7322–7327. https://doi.org/10.1128/AEM.01767-10
Anbu P, Kang C, Shin Y, So J (2016) Formation of calcium carbonate minerals by bacteria and its multiple applications. Springer Plus 5(250):1–26. https://doi.org/10.1186/s40064-016-1869-2
Sarayu K, Iyer NR, Murthy AR (2014) Exploration on the biotechnological aspect of the ureolytic bacteria for the production of the cementitious materials:a review. Appl Biochem Biotechnol 172:2308–2323. https://doi.org/10.1007/s12010-013-0686-0
Benzerara K, Miot J, Morin G, Ona-Nguema G, Skouri-Panet F, Férard C (2011) Significance, mechanisms and environmental implications of microbial biomineralization. C R Geosci 343:160–167
Dhami NK, Reddy MS, Mukherjee A (2013) Biomineralization of calcium carbonates and their engineered applications: a review. Front Microbiol 4:1–13. https://doi.org/10.3389/fmicb.2013.00314
Mahanty B, Kim S, Kim CG (2013) Assessment of a biostimulated or bioaugmented calcification system with bacillus pasteurii in a simulated soil environment. Microb Ecol 65:679–688. https://doi.org/10.1007/s00248-012-0137-4
Krajewska B (2018) Urease-aided calcium carbonate mineralization for engineering applications: a review. J Adv Res 13(2018):59–67. https://doi.org/10.1016/j.jare.2017.10.009
Weaver TJ, Burbank M, Lewis A, Lewis R, Crawford R, Williams B (2011) Bio-induced calcite, iron, and manganese precipitation for geotechnical engineering applications. In: Proceedings of geo-frontiers 2011: advances in geotechnical engineering, Dallas TX, ASCE, Geotechnical Special Publication, vol 211, pp 3975–3983
Harkes MP, van Paassen LA, Booster JL, Whiffin VS, van Loosdrecht MCM (2010) Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol Eng 36(2):112–117. https://doi.org/10.1016/j.ecoleng.2009.01.004
Godleads OA, Prekeyi TF, Samson EO, Igelenyah E (2015) Bioremediation, biostimulation and bioaugmentation: a review. Int J Environ Bioremediat Biodegrad 3(1):28–39. https://doi.org/10.12691/ijebb-3-1-5
Tamayo-Figueroa DP, Castillo E, Brandão PFB (2019) Metal and metalloid immobilization by microbiologically induced carbonates precipitation. World J Microbiol Biotechnol 35:58. https://doi.org/10.1007/s11274-019-2626-9
Torres-Aravena ÁE, Duarte-Nass C, Azócar L, Mella-Herrera R, Rivas M, Jeison D (2018) Can microbially induced calcite precipitation (MICP) through a ureolytic pathway be successfully applied for removing heavy metals fromwastewaters? Crystals 8:438. https://doi.org/10.3390/cryst8110438
Elektorowicz M (1994) Bioremediation of petroleum-contaminated clayey soil with pretreatment. Environ Technol 15(4):373–380. https://doi.org/10.1080/09593339409385440
Piehler MF, Swistak JG, Pinckney JL, Paerl HW (1999) Stimulation of diesel fuel biodegradation by indigenous nitrogen fixing bacterial consortia. Microb Ecol 38:69–78. https://doi.org/10.1007/s002489900157
Rhykerd RL, Crews B, McInnes KJ, Weaver RW (1999) Impact of bulking agents, forced aeration and tillage on remediation of oil-contaminated soil. Bioresour Technol 67:279–285
Chen M, Shih K, Hu M, Li F, Liu C, Wu W, Tong H (2012) Biostimulation of indigenous microbial communities for anaerobic transformation of pentachlorophenol in paddy soils of Southern China. J Agric Food Chem 60:2967–2975. https://doi.org/10.1021/jf204134w
Kanissery RG, Sims GK (2011) Biostimulation for the enhanced degradation of herbicides in soil. Appl Environ Soil Sci. https://doi.org/10.1155/2011/843450
Gat D, Tsesarsky M, Shamir D (2011) Ureolytic calcium carbonate precipitation in the presence of non-ureolytic competing bacteria. In: Proceedings of geo-frontiers 2011: advances in geotechnical engineering, Dallas TX, ASCE, Geotechnical Special Publication, vol 211, pp 3966–3974
LaRock PA, Donovan LS (2001) Survival of a hydrocarbon-utilizing bacterium when introduced into native and foreign environments. OSC Study MMS 2001-094, U.S. Dept. of the Interior, Minerals Management Service, Gulf of Mexico OSC Region, New Orleans
Burbank M, Weaver T, Lewis R, Williams T, Williams B, Crawford R (2013) Geotechnical tests of sands following bioinduced calcite precipitation catalysed by indigenous bacteria. J Geotech Geoenviron Eng 139(6):928–936. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000781
Burbank MB, Weaver TJ, Green TL, Williams T, Williams BC, Crawford RL (2011) Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol J 28(4):301–312. https://doi.org/10.1080/01490451.2010.499929
Dawoud O, Chen CY, Soga K (2014) Microbial induced calcite precipitation (MICP) using surfactants. In: Proceedings of geo-congress 2014: technical papers, ASCE, Geotechnical Special Publication, vol 234, pp 1635–1643
Fujita Y, Ferris FG, Lawson RD, Colwell FS, Smith RW (2000) Subscribed content calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiol J 17:305–318. https://doi.org/10.1080/782198884
Rodriguez-Navarro C, Rodriguez-Gallego M, Ben Chekroun K, Gonzalez-Munoz MT (2003) Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl Environ Microbiol 69:2182–2193. https://doi.org/10.1128/AEM.69.4.2182-2193.2003
Dupraz C, Visscher PT, Baumgartner LK, Reid RP (2004) Microbe-mineral interactions: early carbonate precipitation in a hyper-saline lake (Eleuthera Island, Bahamas). Sedimentology 51:745–765. https://doi.org/10.1111/j.1365-3091.2004.00649.x
Reeburgh WS (2007) Oceanic methane biogeochemistry. Chem Rev 107:486–513. https://doi.org/10.1021/cr050362v
Ersan YÇ, de Belie N, Boon N (2015) Microbially induced CaCO3 precipitation through denitrification: an optimization study in minimal nutrient environment. Biochem Eng J 101(2015):108–118. https://doi.org/10.1016/j.bej.2015.05.006
Zhu T, Dittrich M (2016) Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front Bioeng Biotechnol 4:4. https://doi.org/10.3389/fbioe.2016.00004
Mujah D, Shahin MA, Cheng L (2017) State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol J. https://doi.org/10.1080/01490451.2016.1225866
Hommel J, Lauchnor E, Gerlach R, Cunningham AB, Ebigbo A, Helmig R, Class H (2016) Investigating the influence of the initial biomass distribution and injection strategies on biofilm-mediated calcite precipitation in porous media. Transp Porous Media 114:557. https://doi.org/10.1007/s11242-015-0617-3
Baumgartner LK, Reid RP, Dupraz C, Decho AW, Buckley DH, Spear JR, Przekop KM, Visscher PT (2006) Sulfate reducing bacteria in microbial mats: changing paradigms, new discoveries. Sediment Geol 185(2006):131–145. https://doi.org/10.1016/j.sedgeo.2005.12.008
Paul VG, Wronkiewicz DJ, Mormile MR (2017) Impact of elevated CO2 concentrations on carbonate mineral precipitation ability of sulfate-reducing bacteria and implications for CO2 sequestration. Appl Geochem 78:250–271. https://doi.org/10.1016/j.apgeochem.2017.01.010
González-Muñoz MT, Rodriguez-Navarro C, Martínez-Ruiz F, Arias JM, Merroun ML, Rodriguez-Gallego M (2010) Bacterial biomineraliza-tion: new insights from Myxococcus-induced mineral precipitation. Geol Soc Spec Publ 336:31–50. https://doi.org/10.1144/SP336.3
Seifan M, Berenjian A (2019) Microbially induced calcium carbonate precipitation: a widespread phenomenon in the biological world. Appl Microbiol Biotechnol 103:4693–4708. https://doi.org/10.1007/s00253-019-09861-5
Achal V, Pan X (2011) Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation. Curr Microbiol 62:894–902. https://doi.org/10.1007/s00284-010-9801-4
Achal V, Pan X (2014) Influence of calcium sources on microbially induced calcite precipitation calcite precipitation by Bacillus sp. CR2. Appl Biochem Biotechnol 173:307–317. https://doi.org/10.1007/s12010-014-0842-1
Morales L, Romero E, Jommi C, Garzón E, Giménez A (2015) Ageing effects on the small-strain stiffness of a biotreated compacted soil. Géotech Lett. https://doi.org/10.1680/jgele.15.00044
Morales L, Romero E, Jommi C, Garzón E, Giménez A (2015) Feasibility of a soft biological improvement of natural soils used in compacted linear earth construction. Acta Geotech 10:157–171. https://doi.org/10.1007/s11440-014-0344-x
Fujita Y, Taylor T, Gresham T, Delwiche M, Colwell F, Mcling T, Petzke LM, Smith RTW (2008) Stimulation of microbial urea hydrolysis in groundwater to enhance calcite precipitation. J Environ Sci Technol 42(8):3025–3032. https://doi.org/10.1021/es702643g
Tiano P, Biagiotti L, Mastromei G (1999) Bacterial bio-mediated calcite precipitation for monumental stones conservation: methods of evaluation. J Microbiol Methods 36:139–145. https://doi.org/10.1016/S0167-7012(99)00019-6
De Muynck W, Debrouwer D, De Belie N, Verstraete W (2008) Bacterial carbonate precipitation improves the durability of cementitious materials. Cem Concr Res 38:1005–1014. https://doi.org/10.1016/j.cemconres.2008.03.005
Bang SS, Lippert JJ, Yerra U, Mulukutla S, Ramakrishnan V (2010) Microbial calcite, a bio-based smart nanomaterial in concrete remediation. Int J Smart Nano Mater 1(1):28–39. https://doi.org/10.1080/19475411003593451
Van Tittelboom K, De Belie N, De Muynck W, Verstraete W (2010) Use of bacteria to repair cracks in concrete. Cem Concr Res 40:157–166
Chahal N, Rajor A, Siddique R (2011) Calcium carbonate precipitation by different bacterial strains. Afr J Biotechnol 10(42):8359–8372. https://doi.org/10.5897/AJB11.345
Silva FB, De Belie N, Boon N, Verstraete W (2015) Production of non-axenic ureolytic spores for self-healing concrete applications. Constr Build Mater 93:1034–1041. https://doi.org/10.1016/j.conbuildmat.2015.05.049
Zhu T, Paulo C, Merroun ML, Dittrich M (2015) Potential application of biomineralization by Synechococcus PCC8806 for concrete restoration. Ecol Eng 82:459–468. https://doi.org/10.1016/j.ecoleng.2015.05.017
Chen H, Qian C, Huang H (2016) Self-healing cementitious materials based on bacteria and nutrients immobilized respectively. Constr Build Mater 126:297–303. https://doi.org/10.1016/j.conbuildmat.2016.09.023
Ersan YÇ, Hernandez-Sanabria E, Boon N, de Belie N (2016) Enhanced crack closure performance of microbial mortar through nitrate reduction. Cem Concr Compos 70:159–170. https://doi.org/10.1016/j.cemconcomp.2016.04.001
Akimana RM, Bista H, Seo Y, Li L, Howard LJ, Dewoolkar MM, Hu L (2016) Multi-scale experimental and numerical study of microbially-induced calcite precipitation in sandy soils: preliminary evidence and observations. In: Proceedings of geo-China 2016: technical papers, ASCE, Geotechnical Special Publication, vol 263, pp 77–84
Chang I, Im J, Cho G (2016) An environmentally-friendly geotechnical approach for soil erosion reduction using microbial biopolymers. In: Proceedings of geo-Chicago 2016: technical papers, ASCE, Geotechnical Special Publication, vol 269, pp 17–24
Farah T, Souli H, Fleureau J, Kermouche G, Fry J, Girard B, Aelbrecht D, Lambert J, Harkes M (2016) Durability of bioclogging treatment of soils. J Geotech Geoenviron Eng 142(9):04016040. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001503
Stabnikov V, Jian C, Ivanov V, Li Y (2013) Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World J Microbiol Biotechnol 29:1453–1460. https://doi.org/10.1007/s11274-013-1309-1
Tirkolaei HK, Bilsel H (2015) Statistical modeling of environmental factors on microbial ureolysis process for biocement production. Adv Mater Sci Eng 2015:1–14. https://doi.org/10.1155/2015/340930
Amarakoon GGNN, Kawasaki S (2016) Factors affecting the improvement of sand properties treated with microbially- induced calcite precipitation. In: Proceedings of geo-Chicago 2016: technical papers, ASCE, Geotechnical Special Publication, vol 269, pp 72–83
Li L, Li M, Ogbonnaya U, Wen K, Tian A, Amini F (2016) Influence of fiber addition on mechanical properties of MICP-treated sand. J Mater Civ Eng 28(4):04015166. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001442
Li L, Li M, Ogbonnaya U, Wen K, Li C, Amini F (2016) Experimental investigation of the mechanical properties of MICP-treated sands reinforced with discrete randomly distributed fiber. In: Proceedings of geo-Chicago 2016: technical papers, ASCE, Geotechnical Special Publication, vol 269, pp 52–61
Xu J, Du Y, Jiang Z, She A (2015) Effects of calcium source on biochemical properties of microbial CaCO3 precipitation. Front Microbiol 6:1366. https://doi.org/10.3389/fmicb.2015.01366
Kile DE, Eberl AR, Hoch AR, Reddy MM (2000) An assessment of calcite crystal growth mechanisms based on crystal size distribution. J Geochim Cosmochim Acta 64:2937–2950
Hammes F, Verstraete W (2002) Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol 1:3–7. https://doi.org/10.1023/A:1015135629155
Gurbuz A, Sari YD, Yuksekdag ZN, Cinar B (2011) Cementation in a matrix of loose sandy soil using biological treatment method. Afr J Biotechnol 10(38):7432–7440. https://doi.org/10.5897/AJB11.379
Mortenson BM, Haber MJ, Dejong JT, Caslake LF, Nelson DC (2011) Effects of environmental factors on microbial-induced calcite precipitation. Appl Microbiol 111(2):338–349. https://doi.org/10.1111/j1365-2672-2011.05065x
Handley-Sidhu S, Sham E, Cuthbert MO, Nougarol S, Mantle M, Johns ML, Macaskie LE, Renshaw JC (2013) Kinetics of urease-mediated calcite precipitation and permeability reduction of porous media evidenced by magnetic resonance imaging. Int J Environ Sci Technol 10:881–890. https://doi.org/10.1007/s13762-013-0241-0
Soon N, Lee L, Khun T, Ling H (2014) Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. J Geotech Geoenviron Eng 140(5):04014006. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001089
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36:118–136. https://doi.org/10.1016/j.ecoleng.2009.02.006
Whiffin V, van Paassen LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24(5):417–423
Dupraz S, Parmetier M, Menez B, Guyot F (2009) Experimental and numerical modeling of bacterially induced pH increase and calcite precipitation in saline aquifers. Chem Geol 265:44–53. https://doi.org/10.1016/j.chemgeo.2009.05.003
Fujita Y, Redden GD, Ingram JC, Cortez MM, Ferris FG, Smith RW (2004) Strontium incorporation into calcium carbonate generated by microbial ureolysis. Geochim Cosmochim Acta 65(15):3261–3270. https://doi.org/10.1016/j.gca.2003.12.018
Ferris FG, Phoenix V, Fujita Y, Smith RW (2004) Kinetics of calcite precipitation Induced by ureolytic bacteria at 10 to 20 °C in artificial groundwater. Geochim Cosmochim Acta 68(8):1701–1722
Whiffin V (2004) CaCO3 precipitation for the production of biocement. Doctoral dissertation, Murdoch Univ., Perth, Western Australia
Sun X, Miao L, Tong T, Wang C (2018) Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. https://doi.org/10.1007/s11440-018-0758-y
Inagaki Y, Tsukamoto M, Mori H, Sasaki T, Soga K, Qabany A, Taha T (2011) The influence of injection conditions and soil types on soil improvement by microbial functions. In: Proceedings of geo-Frontiers 2011: advances in geotechnical engineering, Dallas TX, ASCE, Geotechnical Special Publication, vol 211, pp 4021–4030
Okwadha GD, Li J (2010) Optimum conditions for microbial carbonate precipitation. Chemosphere 81:1143–1148. https://doi.org/10.1016/j.chemosphere.2010.09.066
Keykha HA, Asadi A, Huat BBK, Kawasaki S (2018) Laboratory conditions for maximal calcium carbonate precipitation induced by Sporosarcina pasteurii and Sporosarcina aquimarina bacteria. Environ Geotech 2018:1–5. https://doi.org/10.1680/jenge.16.00009
Neupane D, Yasuhara H, Kinoshita N, Unno T (2013) Applicability of enzymatic calcium carbonate precipitation as a soil-strengthening technique. J Geotech Geoenviron Eng 139(12):2201–2211. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000959
Osinubi KJ, Yohanna P, Eberemu AO, Ijimdiya TS (2019) Evaluation of hydraulic conductivity of lateritic soil treated with Bacillus Coagulans for use in waste containment applications. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, vol 3. Springer, Hangzhou, pp 401–409. https://doi.org/10.1007/978-981-13-2227-3_50
Osinubi KJ, Sani JE, Eberemu AO, Ijimdiya TS, Yakubu SE (2019) Unconfined compressive strength of Bacillus Pumilus treated lateritic soil. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, vol 3. Springer, Hangzhou, pp 410–418. https://doi.org/10.1007/978-981-13-2227-3_51
Osinubi KJ, Gadzama EW, Eberemu AO, Ijimdiya TS, Yakubu SE (2019) Evaluation of the strength of compacted lateritic soil treated with Sporosarcina pasteurii. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, vol 3. Springer, Hangzhou, pp 419–428. https://doi.org/10.1007/978-981-13-2227-3_52
Oliveira PJV, Neves JPG (2019) Effect of organic matter content on enzymatic biocementation process applied to coarse-grained soils. J Mater Civ Eng 31(7):04019121. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002774
van Paassen LA, Harkes MP, van Zwieten GA, van der zon WH, van der Star WRL, van Loosdrecht MCM (2009) Scale-up of Biogrout: a biological reinforcement method. In: Proceedings of the 17th international conference on soil mechanics and geotechnical engineering, pp 2328–2333. https://doi.org/10.3233/978-1-60750-031-5-2328
Tobler DJ, Cuthbert MO, Greswell RB, Riley MS, Renshaw JC, Handley-Sidhu S, Phoenix VR (2011) Comparison of rates of ureolysis between Sporosarcina pasteurii and an indigenous groundwater community under conditions required to precipitate large volumes of calcite. Geochim Cosmochim Acta 75:3290–3301. https://doi.org/10.1016/j.gca.2011.03.023
Siddique R, Chahal NK (2011) Effect of ureolytic bacteria on concrete properties: review. Constr Build Mater 25:3791–3801. https://doi.org/10.1016/j.conbuildmat.2011.04.010
Harikrishnan H, Kadaikunnan S, Moorthy IG, Anuf AR, Ponmurugan K, Kumar RS (2015) Improvement of concrete durability by bacterial carbonate precipitation. South Indian J Biol Sci 1(2):90–96
Junfeng T, Yu Z, Yuanyuan R, Xiaoyu L (2016) Development of microbially induced calcium carbonate precipitation technology in soil improvement. Int J Eng Adv Res Technol 2(3):26–29
Nguyen TH, Ghorbel E, Fares H, Cousture A (2019) Bacterial self-healing of concrete and durability assessment. Cem Concr Compos 104:103340. https://doi.org/10.1016/j.cemconcomp.2019.103340
Ivanov V, Stabnikov V, Kawasaki S (2019) Ecofriendly calcium phosphate and calcium bicarbonate biogrouts. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.01.315
Hammes F, Boon N, de Villiers J, Verstraete W, Siciliano SD (2003) Strain-specific ureolytic microbial calcium carbonate precipitation. Appl Environ Microbiol 69(8):4901–4909. https://doi.org/10.1128/AEM.69.8.4901-4909.2003
Mitchell AC, Ferris FG (2005) the coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: temperature and kinetic dependence. Geochim Cosmochim Acta 69(17):4199–4210. https://doi.org/10.1016/j.gca.2005.03.014
Mitchell AC, Dideriksen K, Spangler LH, Cunningham AB, Gerlach R (2010) Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ Sci Technol 44:5270–5276. https://doi.org/10.1021/es903270w
Achal V, Pan X, Zhang D, Fu Q (2012) Bioremediation of Pb-contaminated soil based on microbially induced calcite precipitation. J Microbiol Biotechnol 22(2):244–247. https://doi.org/10.4014/jmb.1108.08033
Achal V, Pan X, Zhang D (2012) Bioremediation of strontium (Sr) contaminated aquifer quartz sand based on carbonate precipitation induced by Sr resistant Halomonas sp. Chemosphere 89:764–768. https://doi.org/10.1016/j.chemosphere.2012.06.064
Achal V, Pan X, Lee D, Kumari D, Zhang D (2013) Remediation of Cr(VI) from chromium slag by biocementation. Chemosphere 93:1352–1358. https://doi.org/10.1016/j.chemosphere.2013.08.008
Lauchnor EG, Schultz LN, Bugni S, Mitchell AC, Cunningham AB, Gerlach R (2013) Bacterially induced calcium carbonate precipitation and strontium coprecipitation in a porous media flow system. Environ Sci Technol 47:1557–1564. https://doi.org/10.1021/es304240y
Kang C, Han S, Shin Y, Oh SJ, So J (2014) Bioremediation of Cd by microbially induced calcite precipitation. Appl Biochem Biotechnol 172:1929–1937. https://doi.org/10.1007/s12010-013-0626-z
Kang C, Han S, Shin Y, Oh SJ, So J (2014) Microbially induced calcite precipitation-based sequestration of strontium by Sporosarcina pasteurii WJ-2. Appl Biochem Biotechnol 174:2482–2491. https://doi.org/10.1007/s12010-014-1196-4
Kang C, Oh SJ, Shin Y, Han S, Nam I, So J (2015) Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol Eng 74:402–407. https://doi.org/10.1016/j.ecoleng.2014.10.009
Kang C, Kwon Y, So J (2016) Bioremediation of heavy metals by using bacterial mixtures. Ecol Eng 89:64–69. https://doi.org/10.1016/j.ecoleng.2016.01.023
Nam I, Chon C, Jung K, Choi S, Choi H, Park S (2015) Calcite precipitation by ureolytic plant (Canavalia ensiformis) extracts as effective biomaterials. J Civ Eng 19(6):1620–1625. https://doi.org/10.1007/s12205-014-0558-3
Rodrigues JD, Pinto APF (2019) Stone consolidation by biomineralisation. Contribution for a new conceptual and practical approach to consolidate soft decayed limestones. J Cult Herit 39:82–92. https://doi.org/10.1016/j.culher.2019.04.022
Yasodian SE, Dutta RK, Mathew L, Anima TM, Seena SB (2012) Effect of microorganism on engineering properties of cohesive soils. Geomech Eng 4(2):135–150
Chittoori BCS, Burbank M, Islam MT (2018) Evaluating the effectiveness of soil-native bacteria in precipitating calcite to stabilize expansive soils. In: IFCEE 2018: innovations in ground improvements for soils, pavements, and subgrades: selected papers from sessions of the international foundation congress and equipment expo 2018: 5–10 Mar 2018 Orlando, Florida. American Society of Civil Engineers (ASCE). https://doi.org/10.1061/9780784481592.007
Osinubi KJ, Eberemu AO, Gadzama EW, Ijimdiya TS (2019) Plasticity characteristics of lateritic soil treated with Sporosarcina pasteurii in microbial-induced calcite precipitation application. SN J Appl Sci 1:829. https://doi.org/10.1007/s42452-019-0868-7
Li M, Fu Q, Zhang Q, Achal V, Kawasaki S (2015) Bio-grout based on microbially induced sand solidification by means of Asparaginase activity. Sci Rep 5(16128):1–9. https://doi.org/10.1038/srep16128
Yasuhara H, Hayashi K, Okamura M (2011) Evolution in mechanical and hydraulic properties of calcite-cemented sand mediated by biocatalyst. In: Proceedings of geo-frontiers 2011: advances in geotechnical engineering, Dallas TX, ASCE, Geotechnical Special Publication, vol 211, pp 3984–3992
Gomez M, Anderson CM, Dejong JT, Nelson DC, Lau XH (2014) Bacteria, bio-films and invertebrates… the next generation of geotechnical engineers. In: Proceedings of geo-congress 2014: technical papers, ASCE, Geotechnical Special Publication, vol 234, pp 1674–1682
Dove JE, Shillaber CM, Becker TS, Wallace AF, Dove PM (2011) Biologically inspired silicification process for improving mechanical properties of sand. J Geotech Geoenviron Eng 137(10):949–957. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000497
Li B, Chu J, Whittle A (2016) Biotreatment of fine-grained soil through the bioencapsulation method. In: Proceedings of geo-Chicago 2016: technical papers, ASCE, Geotechnical Special Publication, vol 269, pp 25–32
Putra H, Yasuhara H, Kinoshita N, Neupane D, Lu CW (2016) Effect of magnesium as substitute material in enzyme-mediated calcite precipitation for soil-improvement technique. Front Bioeng Biotechnol 4:37. https://doi.org/10.3389/fbioe.2016.00037
Salifu E, MacLachlan E, Iyer KR, Knapp CW, Tarantino A (2016) Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: a preliminary investigation. Eng Geol 201:96–105. https://doi.org/10.1016/j.enggeo.2015.12.027
Jijian L, Hongyin X, Xiaoqing H, Yue Y, Dengfeng F, Shuwang Y, Hao Q (2018) Biogrouting of hydraulic fill fine sands for reclamation projects. Mar Georesour Geotechnol. https://doi.org/10.1080/1064119X.2017.1420115
Aamir M, Abdelmalek B, Will PG (2018) Improvement of coarse sand engineering properties by microbially induced calcite precipitation. Geomicrobiol J. https://doi.org/10.1080/01490451.2018.1488019
Osinubi KJ, Eberemu AO, Ijimdiya ST, Yakubu SE, Sani JE (2017) Potential use of B. Pumilus in microbial-induced calcite precipitation improvement of lateritic soil. In: Proceedings of the 2nd symposium on coupled phenomena in environmental geotechnics (CPEG2), Leeds, United Kingdom, 6–8 September 2017. Session: Clean-ups, Paper #64, pp 1–6
Mitchell JK, Jaber M (1990) Factors controlling the long-term properties of clay liners. In: Geotechnical Special Publication no. 25. ASCE, pp 84–105
Li B, Li LY, Grace JR (2015) Adsorption and hydraulic conductivity of landfill-leachate perfluorinated compounds in bentonite barrier mixtures. J Environ Manag 156:236–243. https://doi.org/10.1016/j.jenvman.2015.04.003
Dekuyer A, Cheng L, Shahin MA, Cord-Ruwisch R (2012) Calcium carbonate induced precipitation for soil improvement by urea hydrolysing bacteria. In: Advances in civil, environmental, and materials research (ACEM’ 12), Seoul, Korea, 26–30 August 2012
Soon N, Lee L, Khun T, Ling H (2013) Improvements in engineering properties of soils through microbial-induced calcite precipitation. J Civ Eng 17(4):718–728. https://doi.org/10.1007/s12205-013-0149-8
Osinubi KJ, Gadzama EW, Eberemu AO, Ijimdiya TS (2018) Influence of Sporosarcina pasteurii induced precipitate on the hydraulic conductivity of lateritic soil compacted with reduced standard proctor energy. Faculty of Engineering Ahmadu Bello University Zaria, National Engineering Conference 2018. “The Role of Engineering in the Diversification of Nigerian Economy” 14th–17th November 2018, Ahmadu Bello University, Zaria, pp 109–114
Wiszniewski M, Cabalar AF (2014) Hydraulic Conductivity of a biopolymer treated sand. In: New frontiers in geotechnical engineering. Geotechnical Special Publication no 243, ASCE, pp 19–27
Carrel M, Morales VL, Beltran MA, Derlon N, Kaufmann R, Morgenroth E, Holzner M (2018) Biofilms in 3D porous media: delineating the influence of the pore network geometry, flow and mass transfer on biofilm development. Water Res. https://doi.org/10.1016/j.watres.2018.01.059
Daniel DE, Benson CH (1990) Water content density criteria for compacted soil liners. J Geotech Eng 116(12):1811–1830
Pal S, Rakshit S, Mukherjee SN, Ghosh S (2017) Attenuation of phenol from aqueous solutions using fine-grained soils: experimental design using a statistical approach. J Hazard Toxic Radioact Waste 21(3):04017002–04017009. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000352
Daniel DE, Wu YK (1993) Compacted clay liners and covers for arid site. J Geotech Eng 119(2):223–237
Albrecht BA, Benson CH (2001) Effect of desiccation on compacted natural clay. J Geotech Geoenviron Eng 127(1):67–75
Edil TB, Linda KS, Berthouex PM (1992) Interaction of inorganic leachate with compacted pozzolanic fly ash. J Geotech Eng 118(9):1410–1430
Shackelford CD, Kristin M (2014) Hydraulic conductivity and compatibility of bentonite for hydraulic containment barriers. In: GeoCongress, Atlanta Georgia, ASCE (From soil behavior fundamentals to innovations in Geotechnical Engineering), pp 370–386
Osinubi KJ, Eberemu AO, Ijimdiya TS, Gadzama EW, Yakubu SE (2018) Evaluation of the strength of compacted lateritic soil treated with Sporosarcina pasteurii-induced precipitate. In: 2018 Proceedings of the Nigerian building and road research institute international conference. Theme: Sustainable Development Goals (SDGs) and the Nigerian Construction Industry—Challenges and the Way Forward, 12–14 June 2018, Abuja, Nigeria, pp 383–391
Osinubi KJ, Eberemu AO, Ijimdiya TS, Sani JE, Yakubu SE (2018) Volumetric shrinkage of compacted lateritic soil treated with bacillus pumilus. In: Farid A, Chen H (eds) Proceedings of GeoShanghai 2018 international conference: geoenvironment and geohazard, (GSIC). Springer, Singapore, pp 483–490. https://doi.org/10.1007/978-981-13-0128-5_36
Osinubi KJ, Gadzama EW, Eberemu AO, Ijimdiya TS (2018) Volumetric shrinkage of compacted lateritic soil treated with Sporosarcina pasteurii. In: Proceedings of the 1st international civil engineering conference (ICEC 2018). Infrastructure development in the context of contemporary economic challenges 7th–9th November 2018. Federal University of Technology, Minna, pp 184–193
Osinubi KJ, Gadzama EW, Eberemu AO, Ijimdiya TS (2019) Desiccation induced shrinkage of microbial-induced calcite precipitate treated compacted lateritic soil. In: Book of abstract of University of Ibadan, Department of Civil Engineering Conference on Sustainable Construction for National Development. Theme: Sustainable construction for national development, 10th–12th July 2019, Ibadan, Nigeria, p 20
Osinubi KJ, Eberemu AO, Ijimdiya TS, Gadzama EW, Yohanna P (2019) Compatibility of landfill leachate with compacted lateritic soil treated with bacillus coagulans and Sporosarcina pasteurii using microbial induced calcite precipitation technique. In: 2019 Nigerian building and road research institute international conference. Theme: Construction practices in Nigeria: issues, prospects and solutions, 2–4 July 2019, Abuja, Nigeria
Osinubi KJ, Eberemu AO, Ijimdiya TS, Yohanna P (2020) Interaction of landfill leachate with compacted lateritic soil treated with bacillus coagulans using microbial-induced calcite precipitation approach. J Hazard Toxic Radioact Waste 24(1):040190249. https://doi.org/10.1061/(asce)hz.2153-5515.0000465
Tang Q, Gu F, Zhang Y, Zhang Y, Mo J (2018) Impact of biological clogging on the barrier performance of landfill liners. J Environ Manag 222(2018):44–53. https://doi.org/10.1016/j.jenvman.2018.05.039
Zhang Y, Pan L, Fei W, Ning Z, Tang Q (2019) Estimation of vertical barrier performance based on microbial improvement. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, 28th October–1st November, Hangzhou, China, vol 2. Springer, Singapore, pp 300–307. https://doi.org/10.1007/978-981-13-2224-2_37
Wirth X, Yeboa NNN, Burns S (2019) Lead adsorption by biomass and weathered coal fly ashes. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics (ICEG 2018). Towards a sustainable geoenvironment, 28th October–1st November, Hangzhou, China. Springer, Singapore, pp 367–373. https://doi.org/10.1007/978-981-13-2221-1_37
Muhammed AS, Kassim KA, Zango MU (2018) Review on biological process of soil improvement in the mitigation of liquefaction in sandy soil. In: MATEC web of conferences, vol 250, p 01017. https://doi.org/10.1051/matecconf/201825001017
O’Donnell ST, Rittmann BE, Kavazanjian E (2017) MIDP: liquefaction mitigation via microbial denitrification as a two-stage process. I: desaturation. J Geotech Geoenviron Eng 143(12):04017094-11. https://doi.org/10.1061/(asce)gt.1943-5606.0001818
O’Donnell ST, Kavazanjian E, Rittmann BE (2017) MIDP: liquefaction mitigation via microbial denitrification as a two-stage process. II: MICP. J Geotech Geoenviron Eng 143(12):04017095-12. https://doi.org/10.1061/(asce)GT.1943-5606.0001806
Xiao P, Liu H, Xiao Y, Stuedlein AW, Evans TM (2018) Liquefaction resistance of bio-cemented calcareous sand. Soil Dyn Earthq Eng 107(2018):9–19. https://doi.org/10.1016/j.soildyn.2018.01.008
Xiao P, Liu HL, Stuedlein AW, Evans TM, Xiao Y (2019) Effect of relative density and bio-cementation on the cyclic response of calcareous sand. Can Geotech J. https://doi.org/10.1139/cgj-2018-0573
Meyer FD, Bang S, Min S, Stetler LD, Bang SS (2011) Microbiologically-induced soil stabilization: application of Sporosarcina pasteurii for fugitive dust control. In: ASCE geo-frontiers, pp 4002–4011
Anderson J, Bang SPE, Bang SS, Lee SJ, Choi SR, Dho NY (2014) Reduction of wind erosion potential using microbial calcite and soil fibers. In: Geo-congress 2014 technical papers, GSP 234, pp 1664–1673
Jiang N-J, Soga K, Dawoud O (2014) Experimental study of mitigation of soil internal erosion by microbially induced calcite precipitation. In: Geo-congress 2014 technical papers, GSP 234, pp 1586–1593
Maleki M, Ebrahimi S, Asadzadeh F, Tabrizi ME (2016) Performance of microbial-induced calcite precipitation on wind erosion control of sandy soil. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-015-0921-z
Achal V, Pan X, Zhang D (2011) Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol Eng 37:1601–1605. https://doi.org/10.1016/j.ecoleng.2011.06.008
Ma Y, Lin C, Jiang Y, Lu W, Si C, Liu Y (2009) Competitive removal of water-borne copper, zinc and cadmium by a CaCO3-dominated red mud. J Hazard Mater 172(2009):1288–1296. https://doi.org/10.1016/j.jhazmat.2009.07.135
Li M, Cheng X, Guo H (2013) Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int Biodeterior Biodegrad 76(2013):81–85. https://doi.org/10.1016/j.ibiod.2012.06.016
Jain S, Arnepalli DN (2016) Biomineralisation as a remediation technique: a critical review. In: Indian geotechnical conference IGC2016, 15–17 December 2016. IIT Madras, Chennai, India
Cheng L, Cord-Ruwisch R (2012) In situ soil cementation with ureolytic bacteria by surface percolation. Ecol Eng 42:54–72. https://doi.org/10.1016/j.ecoleng.2012.01.013
Karol RH (2003) Chemical grouting and soil stabilization, 3rd edn. M. Dekker, New York
Naeimi M, Haddad A (2018) Investigation on the environmental impact of soil improvement techniques: comparison of cement grouting and biocement. In: Farid A, Chen H (eds) Proceedings of GeoShanghai 2018 international conference: geoenvironment and geohazard (GSIC). Springer, Singapore, pp 483–490. https://doi.org/10.1007/978-981-13-0128-5_53
Pascal S, Niklas H, Christel C, David B, Göran H (2009) Biogrouting compared to jet grouting: environmental (LCA) and economical assessment. J Environ Sci Health Part A 44(4):346–353. https://doi.org/10.1080/10934520802659679
Chiet KTP, Kassim KA, Chen KB, Murtala U, Yah CS, Arefnia A (2016) Effect of reagents concentration on biocementation of tropical residual soil. In: IOP conference series: materials science and engineering, vol 136. https://doi.org/10.1088/1757-899X/136/1/012030
Okyay TO, Rodrigues DF (2015) Biotic and abiotic effects on CO2 sequestration during microbially-induced calcium carbonate precipitation. FEMS Microbiol Ecol 91:fiv017. https://doi.org/10.1093/femsec/fiv017
Kumar K, Dasgupta CN, Nayak B, Lindblad P, Das D (2011) Development of suitable photobioreactors for CO2 sequestration-addressing global warming using green algae and cyanobacteria. Bioresour Technol 102:4945–4953. https://doi.org/10.1016/j.biortech.2011.01.054
De Muynck W, Verbeken K, De Belie N, Verstraete W (2013) Influence of temperature on the effectiveness of a biogenic carbonate surface treatment for limestone conservation. Appl Microbiol Biotechnol 97:1335–1347. https://doi.org/10.1007/s00253-012-3997-0
Alley R, Berntsen T, Bindoff NL, Chen Z, Chidthaisong A, Friedlingstein P et al (2007) Summary for policymakers. In: Solomon S, Qin D, Manning M (eds) Intergovernmental panel on climate change: climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge. www.ipcc.ch/ipccreports/ar4-wg1.htm. Accessed 20 July 2018
Kirk GJD, Versteegen A, Ritz K, Milodowski AE (2015) A simple reactive-transport model of calcite precipitation in soils and other porous media. Geochim Cosmochim Acta 165:108–122. https://doi.org/10.1016/j.gca.2015.05.017
Martinez BC, Barkouki TH, Dejong JD, Ginn TR (2011) Upscaling of microbial induced calcite precipitation in 0.5 m columns: experimental and modelling results. In: Proceedings of geo frontiers in geotechnical engineering 2011: technical papers, ASCE, pp 4049–4059
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osinubi, K.J., Eberemu, A.O., Ijimdiya, T.S. et al. Review of the use of microorganisms in geotechnical engineering applications. SN Appl. Sci. 2, 207 (2020). https://doi.org/10.1007/s42452-020-1974-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-1974-2