Abstract
The melon fruit fly, Zeugodacus cucurbitae (Coquillett) is a quarantine insect pest worldwide and affects the vegetable quality through its direct feeding and indirectly by passing way for secondary pathogens. This study investigated the host susceptibility, preference and offspring performance of Z. cucurbitae under the laboratory conditions. Different vegetable hosts i.e., brinjal (Solanum melongena L.), bitter gourd (Momordica charantia L.), zucchini (Cucurbita pepo L.), bottle gourd (Lagenaria siceraria [Molina] Standley) and cucumber (Cucumis sativus L.) were tested under no choice and free choice tests. Results showed that C. sativus and C. pepo have highest number of visits/host and oviposition puncture/host. C. sativus showed highest pupal recovery and pupal weight in both only choice and free choice test. While, highest percentage of emergence and female off springs were observed in C. pepo under only choice and free choice scenarios. Furthermore, maximum deformities in progeny were observed in case of L. siceraria under both test case scenarios. The current study provides exploratory support that fruit flies respond differently to host species that co-exists in field under choice and no choice test. Further, hosts of advantage to fruit flies are adopted more. The host and choice preference of fruit flies have the influence on the pest management strategies for the vegetable crops.
Similar content being viewed by others
Introduction
The melon fruit fly, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae) is distributed throughout tropical, sub-tropical countries (Fletcher 1987) and considered as federal quarantine pest in Asia and Hawaii (Allwood et al. 1999). Its damage can cause 30 to 100% losses in vegetables depending on environmental conditions and crop susceptibility (Kabir et al. 1991; Gupta and Verma 1992; Dhillon et al. 2005). In Pakistan, where adequate management practices are not being implemented in cucurbits, losses have been estimated at 24% (Stonehouse et al. 1998). Female Z. cucurbitae punctures the soft skin of host with the ovipositor and lays eggs below fruit exocarp (Mir et al. 2014). The actual damage is inflicted by the feeding action of the larval stage which consumes fruit pulp and by burrows inside the fruit, thereby creating entry point for fungal infections. The oviposition marks and presence of larvae as well as secondary infections lower the marketability and value of fruits and vegetables either for local or export markets (Ekesi and Billah 2006; Chen and Ye 2007).
For developing successful management strategies, the knowledge regarding biology, different life stages and host preference of certain insect pests is essential (Mir et al. 2014). The preference- performance hypothesis plays an important role in host use patterns of insects (Khan and Tahira-Binte-Rashid 2011). In documented case studies for insect pests, host performance may vary and could be vital for the host shifting. About 25–40% insects are host specific species (Bush and Butlin 2004). Fruit flies behavior differs in different host plants which influence the selection pressure regarding the biological attributes of insects (Moreau et al. 2017).
Adult female fruit flies are considered to make decisions to oviposit based on the stability of host species for their offspring performance (Kostal 1993; Joachim-Bravo et al. 2001; Fontellas-Brandalha and Zucoloto 2004). In Bactrocera flies, host selection behavior may be affected by skin, size, odor, color, fruit shape foliage characteristics (Brévault and Quilici 2007; Jaleel et al. 2018). Moreover, nutritional level of host can affect the offspring development (Balagawi et al. 2005). In addition, different toxins and resins may also affect the development of fruit flies (Seo et al. 1982; Rattanapun et al. 2009). Usually a broad range of hosts co-exist in field conditions during summer season and the majority of which are susceptible to fruit fly infestation. Previous studies reported oviposition preference and offspring performance of fruit flies based on choice and no-choice trials (Muthuthantri and Clarke 2012; Rauf et al. 2013; Rizk et al. 2014 El-Gendy 2017). The findings of the above mentioned studies demonstrated an oviposition preference hierarchy of fruit flies on host tested. Further, these studies provide a possible role of adult preference for host as an important function in differential oviposition. The current study was conducted to gain detail information to determine whether fruit flies have preference if given choose. Such information is important in determining host susceptibility and fruit fly offspring performance. The current study was therefore conducted to determine oviposition preference of Z. curcubiate on different hosts and scenarios Materials and methods.
In the present study, Z. cucurbitae adults were collected from field area (35°55′11.36”N, 74°22′47.44”E) in Gilgit and kept at Centre for Agriculture and Bioscience International (CABI), Gilgit, Pakistan (35°55′21.3”N 74°21′42.9”E). The fruit fly culture were kept (for 10 generations before the experiments) in perspex rearing cages (60 × 60 × 48 cm) with a cloth sleeve opening at front with a capacity of 1000 female and male flies (1♀/:1♂) and maintained at28 ± 1 °C; 65 ± 5% RH, and photoperiod of L14: D10.. The adult flies were fed on a water based diet consisting of yeast, banana, egg yolk, vitamin B-complex and sugar (unpublished material) and were provided with water on soaked cotton ad libitum. Adult flies were provided with fresh host for laying their eggs. Later, infested vegetables were removed from cages and kept in plastic containers with sand as pupation medium at bottom and kept till pupation. After pupation, pupae were sieved using mesh screen (80) and kept in jars (15 × 6 × 6 cm) till emergence.
Host plants
Different host plants that co-exist with Z. cucurbitae in field at Gilgit including bitter gourd (Momordica charantia L.), zucchini (Cucurbita pepo L.), bottle gourd (Lagenaria siceraria [Molina] Standley), cucumber (Cucumis sativus L.) and brinjal (Solanum melongena L.) were used. The above mentioned hosts were purchased from a local market in Gilgit, Pakistan. Hosts were selected based on no prior infestation or damage. Five to seven days old Z. cucurbitae adults were used for further experimentation while maintaining sex ratio (1:1). Male and female flies were identified according to Drew and Raghu (2002). The adults were provided with the water based diet and water ad libitum. The experiments were conducted at standard laboratory conditions as previously described. Each host preference treatment was replicated thrice for all tests and repeated twice.
Host susceptibility, preference and off spring performance
Choice and no choice test were conducted under labortoray conditions to assess the host susceptibility, preference and off-spring performance of Z. Cucurbitae. No choice test Z. cucurbitae pairs (50) (1♀/:1♂) were placed in plastic jars (15 × 6 × 6 cm) with ~300 g of host plant material (described above) offered separately and left for 48 h. For number of visits per day hosts were observed for 10 h and each host was watched for 2 min/h (Jaleel et al. 2018). Later, exposed hosts were replaced by new hosts in the jars and infested host and oviposition punctures/hosts was counted. Infested hosts were kept separately in plastic jars and sand substratum offered at bottom for pupation later sieved to separate pupae, followed by recording the number of pupae. Later, pupae were weighted in groups and mean pupal weight was calculated (explained in statistical analysis). The pupae were kept in plastic jars for adult emergence, sex ratio and deformity i.e., wings, abdomen and proboscis.
Choice test
For free choice test, Z. cucurbitae pairs (50) (1♀/:1♂) were placed in plastic jars (15 × 6 × 6 cm) with hosts (described above) offered simultaneously after determining the weight of each host (~ 300 g) for egg laying for 48 h. The observation was recorded to investigate efficiency with same parameters as described in “no choice test”.
Statistical analysis
The mean pupal weight (mg) was calculated with aid of following formulae:
The adult flies emergence (%) was calculated using following formulae:
While sex ratio (female) was calculated as by Farooq and Freed (2016) using the following formulae:
The data on means were subjected to Analysis of Variance (ANOVA) to determine means of different parameters i.e., number of visits per day, oviposition puncture/host, recovered pupae, pupal weight, adult emergence, sex ratio and deformity were separated using Tukey’s HSD test value at 0.05% level of significance. All analyses were performed using Statistic 8.1 (McGraw-Hill 2008).
Results
The results regarding host susceptibility and off spring performance attributes i.e., number of visits per day, oviposition puncture/host, number of recovered pupae, pupal weight, adult emergence, deformity and sex ratio of Z. cucurbitae under no choice and free choice tests showed significant variations.
Fruit visits
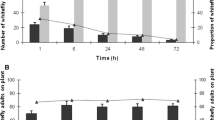
For no choice test, host plant type had a significant effect on number of visits by Z. cucurbitae per day (F4,14 = 11.1, P = 0.001) with C. sativus being visited more time a day(9.67 ± 0.88) followed by C. pepo (8.33 ± 0.33), M. charantia (5.33 ± 1.20), S. melongena (4.00 ± 0.58), and lastly L siceraria (3.00 ± 0.58) s (Fig. 1a). In addition, host plant type also had a significant effect on the oviposition puncture by female flies (F4,14 = 42.0, P = 0.000) with C. sativus had most ovipositional punctures (17.00 ± 1.53) follwed by C. pepo (15.33 ± 0.88) S. melongena (7.33 ± 0.67), M. charantia (6.33 ± 0.67) and lastly L siceraria (3.67 ± 0.33) in no choice test (Fig. 1a).
In case of free choice test, similar trend was observed host plant type had a significant effect on number of visits by Z. cucurbitae (F4,14 = 44.9, P = 0.000) with C. sativus being visited more time (9.33 ± 0.40) followed by C. pepo (8.00 ± 0.58) S. melongena (2.67 ± 0.33), M. charantia (2.67 ± 0.33) and lastly L siceraria (1.33 ± 0.33) (Fig. 1b). In addition, host plant type also had a significant effect on oviposition puncture by female flies (F4,14 = 33.2, P = 0.000) with C. sativus had most ovipositional punctures (14.67 ± 1.45) followed by C. pepo (11.67 ± 1.20), M. charantia (5.00 ± 0.58), S. melongena (4.33 ± 0.33) and lastly L siceraria (2.00 ± 0.58) (Fig. 1b).
Recovered pupae
The results of no choice test showed that host plant type had a significant effect on recovered pupae (F4,14 = 94.4, P = 0.000) with C. sativus had highest pupal recovery (1003.67 ± 57.85) followed by C. pepo (970.0 ± 56.86), M. charantia (669.67 ± 15.38), S. melongena (320.67 ± 15.07) and lastly L. siceraria (187.67 ± 14.68)(Fig. 2a). Similar trend was observed in free choice test as significant effect of host plant was observed on recovered pupae (F4,14 = 64.4, d.f. = 4,14,P = 0.000) with C. sativus (450.33 ± 36.67) had highest pupal recovery followed by C. pepo (370.0 ± 20.84) M. charantia (197.0 ± 19.92), S. melongena (112.0 ± 14.04) and L siceraria (28.67 ± 4.49) (Fig. 2a).
Effect of host plants on different attributes of Zeugodacus cucurbitae under only choice and free choice test, a recovered pupae, b pupal weight (mg), c adult emergence (%) d deformity (%). Means followed by same letters for each column (only choice, free choice test) are not statistically different; HSD, P < 0.05
Pupal weight
Under only choice test, significant effect of host plant was observed on pupal weight (F4,14 = 84.2, P = 0.000) with C. sativus (14.03 ± 0.37) showed maximum pupal weight, followed by C. pepo (13.13 ± 0.20), M. charantia (10.34 ± 0.1) S. melongena (9.32 ± 0.22) while, L. siceraria (8.41 ± 0.34) showed the maximum reduction in it (8.41 ± 0.34) (Fig. 2b). Similar trend was observed in free choice test as results regarding pupal weight was signigcanlty influenced by host type (F4,14 = 230.0, d.f. = 4,14,P = 0.000) with C. sativus (14.15 ± 0.22) (mg) showed maximum pupal weight followed by C. pepo (13.28 ± 0.1), M. charantia (10.17 ± 0.1) S. melongena (9.39 ± 0.18) while, L. siceraria (8.66 ± 0.17) showed the significant reduction in it (8.66 ± 0.17) (mg) (Fig. 2b).
Adult emergence
The data regarding the adult emergence under no choice test showed that adult emergence was significantly influenced by host type (F4,14 = 34.6, d.f. = 4,14, P = 0.000)as C. pepo (90.15 ± 0.70) had the highest adult emergence followed by C. sativus (85.81 ± 1.59), M. charantia (74.54 ± 2.74) S. melongena (72.60 ± 2.86) and lastly L. siceraria (60.52 ± 1.02) (Fig. 2c). While for free choice test, adult emergence was significantly inglunced by host plant type (F4,14 = 33.3, P = 0.000) as highest adult emergence was observed in C. pepo (92.34 ± 1.07) followed by C. sativus (89.39 ± 0.94) M. charantia (81.63 ± 1.07) S. melongena (78.08 ± 1.98) and lastly L. siceraria (64.83 ± 1.07) (Fig. 2c).
Sex ratio
The results regarding sex ratio showed that host plant had significant effect of female percentage under no choice test (F4,14 = 7.36, d.f. = 4,14, P = 0.005) as C. pepo had the highest number of females (52.35 ± 0.38) followed by C. sativus (48.75 ± 1.92), L. siceraria (44.23 ± 1.41), M. charantia (42.98 ± 1.30) and lastly S. melongena (41.14 ± 2.60) (Table 1). In case of free choice test, significant effect on host plant type were observed (F4,14 = 7.36, P = 0.005) as C. pepo showed the highest number of females (52.92 ± 0.87) followed by C. sativus (52.57 ± 1.08), M. charantia (47.58 ± 2.14), S. melongena (45.47 ± 1.60) and lastly L. siceraria (41.19 ± 2.81) (Table 1).
Deformity
The attributes of emerging flies showed significant variations due to host plant type under no choice test (F4,14 = 25.7, d.f. = 4,14, P = 0.000) as L. siceraria (17.54 ± 0.53) showed the maximum deformed flies (combine attributes) followed by M. charantia (11.32 ± 0.77), S. melongena (9.38 ± 1.20), C. sativus (6.99 ± 1.44) and C. pepo (4.510.61) (Fig. 2d). Furthermore, a similar trend for deformed new flies was observed in free choice test (F4,14 = 25.4, P = 0.000) as L. siceraria (14.16 ± 1.17) showed highest deformed flies followed by S. melongena (9.25 ± 0.38), M. charantia (8.03 ± 0.71), C. sativus (5.61 ± 0.94) and C. pepo (3.66 ± 0.50) (Fig. 2d).
Discussion
A significant influence of host plants was observed on the different developmental parameters of the fruit fly. In the current study, Z. cucurbitae preferred C. sativus and C. pepo for oviposition and immature feeding under both no choice and free choice scenarios. The results of number of female visits, oviposition puncture/host and pupal recovery showed higher preference C. sativus > C. pepo > M. charantia > S. melongena > L. siceraria under only choice and free choice test in that order. Our findings suggest all five tested hosts can be utilized by Z. cucurbitae depending upon host availability. Host quality plays a key role on the development of offsprings. Thus both host species and its quality had an influence on the development of immature stages of Z. cucurbitae.
The host preference of fruit flies was determined by different attributes including pupal size, adult emergence percentage, sex ratio and deformity percent under host preference studies. (Li-Li et al. 2008; Rauf et al. 2013). In the present study, C. sativus and C. pepo were most preferred hosts by Z. cucurbitae as shown by high pupal recovery under both scenarios. Similar results were reported by Khan and Tahira-Binte-Rashid (2011) where bitter gourd and cucumber were the most susceptible host for B. cucurbitae as shown by pupal yield recovery. Body sizes of insect being an indicator for insect fitness, large insects are more competitive as frequent mating, high fertility and more dispersion capacity (Navarro-Campos et al. 2011; Thorne et al. 2006). Further, plant species differ in their suitability for serving a food source for insects. In fruit flies host preference is favored by numerous factors including odor, color, shape and size of host (Li-Li et al. 2008; Bruce et al. 2005).
Based on the results of the current study for obtained pupae and adult emergence, it is suggested that Z. cucurbitae can use these natural hosts depending upon their availability and also preference of fruit fly. Similar to the current study, Fitt (1986) noted the fruit fly species abundance on different hosts as female flies make more choices as compared to larval specialism. Although many host plants can provide bursting sustenance of tephritid species, yet host quality administrates the differences in larval specialization and survival rate. The relationship between host preference and offspring performance provides support for the preference- performance hypothesis as female insects will evolve themselves to oviposit more on the hosts which can support their offspring fare better (Akol et al. 2013). Female fruit fly’s response toward a host is influenced by olfactory, visual and contact cues such as the color, size, shape and smell of host fruit (Drew et al. 2003; Brévault and Quilici 2007; Khan and Tahira-Binte-Rashid 2011).
The health of pupae directly influenced the adult emergence while pupa is dependent on the larvae. An adult fails to emerge due to poorly developed pupa. Adult deformity increases as per poor host section and reduces adult emergence (Mayhew 1997). The current study showed that L. siceraria was proved to be a most un-preferable host by Z. cucurbitae signified by the different parameters. The studied vegetables crops showed significant effects on developmental attributes of Z. cucurbitae. The difference in egg laying preference, different pupal weight different sex ratio and deformity of adults on different hosts could be due to number of factors e.g. plant phenology, nutritional, microbial contamination, fruit temperature, peel thickness and maturity of fruits (Feder et al. 1997). Host plants differ in the carbon, nitrogen composition and metabolites which interrupt insect oviposition, feeding, growth and development leading to low fitness and low offspring survival (Roitberg and Isman 1992; Haggstrom and Larsson 1995; Awmack and Leather 2002; Gibbs et al. 2006). Previous studies by Hafsi et al. (2016) explained 30% variability in the larval performance of different fruit fly species was due to water, lipid, carbohydrates and fiber contents of hosts. Moreover, a positive correlation of polyphagous species of fruit flies exhibits for carbohydrate, lipid, and fiber contents while it negatively correlates with water content. For fruit fly species associated with cucurbit hosts a positive correlation with water content and negative correlation with carbohydrate and lipid content exists. The results of previous studies (Zucoloto 1987; Joern and Behmer 1997; Lee et al. 2008; Roeder et al. 2014; Nash and Chapman 2014; Hafsi et al. 2016) showed that carbohydrates were important elements of fruit nutritive value for larvae of tephritid. Host specialization cannot be solely predicted by nutrional measurement as it also affected by secondary metabolites and volatile compounds (Bateman 1972; Renwick 2001). Plant secondary metabolites directly or indirectly influence the fecundity of by being toxigenic or reducing nutrient assimilation (Awmack and Leather 2002). In addition, Erbout et al. (2009) reported that fruits containing high alkaloid concentrations re detrimental for polyphagous tephritid larvae.
The previous experiments on other tephritid have shown that flies adapt behaviorally to the host plants phenology. Several studies have also reported cases where female preference and performance appear uncoupled, or where the relationship is surprisingly weak (Fritz et al. 2000; Faria and Fernandes 2001). Moreover, some hosts were not able to attract flies than others to a greater extent are easier to explain as cuticle proved as a less preferable oviposition substrate (Feng-Ming 1997). The evolutionary and ecological considerations have been proposed to explain apparent mismatches between choice and performance including the fact that the strength of the preference–performance relationship is modified by ecological and / or life-history factors which may have contributed to the observations noted on cucumber in the current studies (Mayhew 2001). All the tested fruit fly species are polyphagous; strictly attacking cucurbit. Within the context selecting for appropriate host, the female flies may encounter several constraints including limitations on the information processing capacity among similar host plant family (Cunningham 2012). The results of current study regarding insect life history on different vegetable hosts might be used for development of management tools against this pest of concern. However, our study did not determine host plant factors that might interrupt with insect infestation and life history attributes. Therefore, further studies are recommended to explore host plant characteristics with respect to environmental conditions. In addition studies regarding behavior, age-stage and two-sex life table parameters for more detailed knowledge is required to achieve a reliable fruit fly management strategy.
Conclusion
In conclusion, these findings provide supporting evidence that Z. cucurbitae prefer C. sativus and C. pepo as hosts. Based on our findings, L. siceraria is less preferred by Z. cucurbitae thus such knowledge may be important in relaxing quarantine restrictors and requirements for post-harvest treatments espically for less preferred host plants.
Change history
20 May 2020
The above mentioned article, was originally published Online First without Open Access. After publication in volume 40, issue 1, page 93���99 the author decided to opt for Open Choice and to make the article an Open Access publication.
References
Akol AM, Masembe C, Isabirye BE, Kukiriza CK, Rwomushana I (2013) Oviposition preference and offspring performance in Phytophagous fruit flies (Diptera: Tephritidae): the African invader, Bactrocera invadens. Int Res J Hort 1:1–14
Allwood AJ, Cninajariyanwong A, Drew RAI, Hamecek EL, Hancock DL, Hengsawad C et al (1999) (1999) host plant records of frut flies (Diptera: Tephritidae) in South Asia. Raffles Bull Zool 47:1–92
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Balagawi S, Vijaysegaran S, Drew RA, Raghu S (2005) Influence of fruit traits on oviposition preference and offspring performance of Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) on three tomato (Lycopersicon lycopersicum) cultivars. Austral Entomol 44:97–103
Bateman M (1972) The ecology of fruit flies. Annu Rev Entomol 17:493–518
Brévault T, Quilici S (2007) Influence of habitat pattern on orientation during host fruit location in the tomato fruit fly, Neoceratitis cyanescens. Bull Entomol Res 97:637–642
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Bush GL, Butlin RK (2004) Sympatric speciation in insects. In: Doebeli M, Metz JAJ, Tautz D (eds) Dieckmann U. Cambridge University Press, Adaptive Speciation, pp 229–248
Chen P, Ye H (2007) Population dynamics of Bactrocera dorsalis (Diptera: Tephritidae) and analysis of factors influencing populations in Baoshanba, Yunnan, China. Entomol Sci 10:141–147
Cunningham JP (2012) Can mechanism help explain insect host choice? J Evol Biol 25:244–251
Dhillon MK, Singh R, Naresh JS, Sharma HC (2005) The melon fruit fly, Bactrocera cucurbitae: a review of its biology and management. J Insect Sci 5:40–56
Drew RAI, Raghu S (2002) The fruit fly fauna (Diptera: Tephritidae: Dacinae) of the rainforest habitat of the Western Ghats, India. Raffles Bull Zool 50:327–352
Drew RAI, Prokopy RJ, Romig MC (2003) Attraction of fruit flies of the genus Bactrocera to colored mimics of host fruit. Entomol Exp Appl 107:39–45
Ekesi S, Billah K (2006) Field guide to the management of economically important tephritid fruit flies in Africa. ICIPE Science Press, Nairobi, p 160
El-Gendy IR (2017) Host preference of the peach fruit Fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae), under laboratory conditions. J Entomol 14:160–167
Erbout N, De Meyer M, Vangestel C, Lens L (2009) Host plant toxicity affects developmental rates in a polyphagous fruit fly: experimental evidence. Biol J Linn Soc 97:728–737
Faria ML, Fernandes GW (2001) Vigour of a dioecious shrub and attack by a galling herbivore. Ecol Entomol 26:37–45
Farooq M, Freed S (2016) Combined effects of Beauveria bassiana (Hypocreales: Clavicipitaceae) and insecticide mixtures on biological parameters of Musca domestica (Diptera: Muscidae). Pakistan J Zool 48:1465–1476
Feder JL, Stolz U, Lewis KM, Perry W, Roethele JB, Rogers A (1997) The effects of winter length on the genetics of apple and hawthorn races of Rhagoletis pomonella (Diptera: Tephritidae). Evolution 51:1862–1876
Feng-Ming L (1997) Ovipositional preference of melon Fly, Bactrocera cucurbitae Coquillett (Diptera: Tephritidae) (I) tests of host plant and color. Chin J Entomol 17:237–243
Fitt G (1986) The roles of adult and larval specializations in limiting the occurrence of five species of Dacus (Diptera: Tephritidae) in cultivated fruits. Oecologia 69:101–109
Fletcher BS (1987) The biology of Dacine fruit flies. Annu Rev Entomol 32:115–144
Fritz RS, Crabb BA, Hochwender CG (2000) Preference and performance of a gallinducing sawfly: a test of the plant vigor hypothesis. Oikos 89:555–563
Fontellas-Brandalha TML, Zucoloto FS (2004) Selection of oviposition sites by wild Anastrepha obliqua (Macquart) (Diptera: Tephritidae) based on the nutritional composition. Neotrop Entomol 33:557–562
Gibbs M, Lace LA, Jones MJ, Moore AJ (2006) Multiple host-plant use may arise from gender-specific fitness effects. J Insect Sci 6:1–8
Gupta D, Verma AK (1992) Population fluctuations of the maggots of fruit flies Dacus cucurbitae Coquillett and D. tau (Walker) infesting cucurbitaceous crops. Adv Plant Sci 5:518–523
Hafsi A, Facon B, Ravigné V, Chiroleu F, Quilici S, Chermiti B, Duyck P-F (2016) Host plant range of a fruit fly community (Diptera: Tephritidae): does fruit composition influence larval performance? BMC Ecol 14. https://doi.org/10.1186/s12898-016-0094-8
Haggstrom H, Larsson S (1995) Slow larval growth on a suboptimal willow results in high predation mortality in the leaf beetle Galerucella lineola. Oecologia 104:308–315
Jaleel W, Tao X, Wang D, Lu L, He Y (2018) Using two-sex life table traits to assess the fruit preference and fitness of Bactrocera dorsalis (Diptera: Tephritidae). J Econ Entomol 20(10):1–10. https://doi.org/10.1093/jee/toy243
Joachim-Bravo IS, Fernandes OA, De-Bortoli SA, Zucoloto FS (2001) Oviposition behavior of Ceratitis capitata Wiedemann (Diptera: Tephritidae): association between oviposition preference and larval performance in individual females. Neotrop Entomol 30:559–564
Joern A, Behmer ST (1997) Importance of dietary nitrogen and carbohydrates to survival, growth, and reproduction in adults of the grasshopper Ageneotettix deorum (Orthoptera: Acrididae). Oecologia 112:201–208
Kabir SMH, Rahman R, Molla MAS (1991) Host plants of Dacinae fruit flies (Diptera: Tephritidae) of Bangladesh. Bangladesh J Entomol 1:60–75
Khan M, Tahira-Binte-Rashid HAJ (2011) Comparative host susceptibility, Oviposition, and colour preference of two Polyphagous Tephritids: Bactrocera cucurbitae (coq.) and Bactrocera tau (Walker). Res J Agric Biol Sci 7:343–349
Kostal V (1993) Physical and chemical factors influencing landing and oviposition by the cabbage root fly on host-plant models. Entomol Exp Appl 66:109–118
Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D (2008) Lifespan and reproduction in Drosophila: new insights from nutritional geometry. PNAS 105:2498–2503
Li-Li R, Li-Yan Q, Qiao-Gen J, Shu-Dong Z, Hua-Guo D (2008) Oviposition preference of oriental fruit fly, Bactrocera dorsalis. Chin. Bull Entomol 4:593–597
Mayhew PJ (1997) Adaptive patterns of host-plant selection by phytophagous insects. Trends Ecol Evol 79:417–428
McGraw-Hill, C., 2008. Statistix 8.1 analytical software. Tallahassee, Florida
Mir SH, Dar SA, Mir GM, Ahmad SB (2014) Biology of Bactrocera cucurbitae (Diptera: Tephritidae) on cucumber. Fla Entomol 97:753–758
Moreau J, Desouhant E, Louâpre P, Goubault M, Rajon E, Jarrige A, Menu F, Thiéry D (2017) How host plant and fluctuating environments affect insect reproductive strategies? In: Sauvion N, Thiéry D, Calatayud P-A (eds) Advances in botanical research. Academic Press Elsevier, USA, pp 259–287
Muthuthantri S, Clarke AR (2012) Five commercial citrus rate poorly as hosts of the polyphagous fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) in laboratory studies. Aust J Entomol 51:289–298
Nash WJ, Chapman T (2014) Effect of dietary components on larval life history characteristics in the medfly (Ceratitis capitata: Diptera, Tephritidae). PLoS One 9:1–9. https://doi.org/10.1371/journal.pone.0086029
Navarro-Campos C, Martínez-Ferrer MT, Campos JM, Fibla JM, Alcaide J, Bargues L, Marzal C, Garcia-Marí F (2011) The influence of host fruit and temperature on the body size of adult Ceratitis capitata (Diptera: Tephritidae) under laboratory and field conditions. Environ Entomol 40:931–938
Rattanapun W, Amornsak W, Clarke AR (2009) Bactrocera dorsalis preference for and performance on two mango varieties at three stages of ripeness. Entomol Exp Appl 131:243–253
Rauf I, Ahmad N, Rashdi SMS, Ismail M, Khan MH (2013) Laboratory studies on ovipositional preference of the peach fruit fly Bactrocera zonata (Saunders) (Diptera: Tephiritidae) for different host fruits. Afr J Agric Res 8:1300–1303
Renwick JAA (2001) Variable diets and changing taste in plant–insect relationships. J Chem Ecol 27:1063–1076
Rizk MMA, Abdel-Galil FA, Temerak SAH, Darwish DYA (2014) Relative preference of peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae) to some fruit and vegetables under laboratory conditions. Arch Phytopathol Plant Protect 47:1376–1380
Roeder KA, Behmer ST, Davidowitz G (2014) Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct Ecol 28:1135–1143
Roitberg BD, Isman MB (1992) Insect chemical ecology. An Evolutionary Approach. Chapman & Hall, New York, p 359
Seo ST, Farias GJ, Harris EJ (1982) Oriental fruit fly: ripening of fruit and its effect on index of infestation of Hawaiian papayas. J Econ Entomol 75:173–178
Stonehouse JM, Mumford JD, Mustafa G (1998) Economic losses to tephritid fruit flies (Diptera: Tephritidae) in Pakistan. Crop Prot 17:159–164
Thorne AD, Pexton JJ, Dytham C, Mayhew PJ (2006) Small body size in an insect shifts development, prior to adult eclosion, towards early reproduction. Proc Biol Sci 273:1099–1103
Zucoloto FS (1987) Feeding habits of Ceratitis capitata (Diptera: Tephritidae): can larvae recognize a nutritionally effective diet? J Insect Physiol 33:349–353
Acknowledgements
We gratefully acknowledge Mr. Riaz Mahmood, CABI’s Biological Control Specialist for providing technical inputs in this study. Acknowledgement is also made to Integrated Pest Management Unit (IPM), Department of Agriculture Extension Gilgit-Baltistan for providing facilitation during this research.
Funding
This study was funded by United States Agency for International Development (USAID) through United States Department of Agriculture (USDA) under the CABI’s project “Phytosanitary Risk Management Program in Pakistan” (PRMP). CABI is an international intergovernmental organisation, and we gratefully acknowledge the core financial support from our member countries (and lead agencies) including the United Kingdom (Department for International Development), China (Chinese Ministry of Agriculture), Australia (Australian Centre for International Agricultural Research), Canada (Agriculture and Agri-Food Canada), Netherlands (Directorate-General for International Cooperation), and Switzerland (Swiss Agency for Development and Cooperation). See http://www.cabi.org/about-cabi/who-we-work-with/key-donors/ for full details.
Author information
Authors and Affiliations
Contributions
MF performed the experiments, analyzed the data wrote the manuscript. SB performed the experiments. SFH and BEB supervised the work, help in data analysis and article writing. FU performed the experiments and aid in the manuscript write up IHS Help in data analysis and article writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farooq, M., Baig, S., Honey, S.F. et al. Evaluation of host susceptibility, preference and offspring performance of Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae) on different hosts. Int J Trop Insect Sci 40, 93–99 (2020). https://doi.org/10.1007/s42690-019-00056-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-019-00056-z