Abstract
Introduction:
The role of CD4+ T cells in the immunopathogenesis of asthma is well documented. Little is known about the role of CD8+ T cells. The aim of this study was to assess peripheral blood subsets of CD4+ and CD8+ T cells expressing naive/memory markers (CD45RA+/RO+) and the activation marker (CD25+) in children with allergic asthma.
Materials and Methods:
Peripheral blood mononuclear cells were isolated from children with allergic asthma and healthy children. T cell subsets were analyzed by flow cytometry for the expressions of CD45RA, CD45RO, and CD25. In this study, some differences in the memory compartment of peripheral blood T cells between asthmatic children and healthy controls were detected.
Results:
The absolute number of CD8+ T cells expressing CD45RO was significantly elevated and the percentages of CD3+ T cells expressing activation marker CD25 and of CD4+ T cells expressing memory marker CD45RO were significantly lower in children with asthma compared with controls. No correlation was found between severity of asthma and peripheral blood lymphocyte subsets.
Conclusions:
There were some differences in the memory compartment of peripheral blood T cells between asthmatic children and healthy controls. The increase in the number of CD8+ T cells expressing the memory marker (CD45RO) in children with allergic asthma may indicate that CD8+ T cells play a role in the pathogenesis of asthma.
Similar content being viewed by others
Introduction
Allergic asthma is one of the most common diseases in childhood that result from both genetic and environmental factors. Numerous studies have underlined the role of activated memory CD4+ T cells as the main producer of Th2 cytokines in asthma and other atopic diseases. Th2 cytokines such as IL-4 and IL-13 interact with resident lung cells, including airway epithelium, myofibroblast, and smooth muscle cells, to induce the asthmatic phenotype [14]. These cytokines contribute to many of the pathophysiological features of asthma, including airway inflammation, mucus secretion, and airway hyperresponsiveness. The production of Th2 cytokines was initially ascribed to CD4+ T cells, but several studies have provided evidence that CD8+ T cells are able to secrete Th2 cytokines and are also essential for allergic inflammation and airway sensitivity [14]. The importance of CD8+ T cells in the pathogenesis and exacerbation of allergic disease has been recently highlighted by the identification of allergen-specific CD8+ T cells [21,]. It is assumed that an increase in activated memory T cells (CD54RO/CD25) in the lung or in peripheral blood may be evidence of chronic inflammation in an asthmatic subject.
This study was performed to compare the expressions of the naive memory marker (CD45RA+/CD45RO+) and the activation marker (CD25+) on peripheral blood T cells between asthmatic children and healthy controls. Inhaled glucocorticosteroids (IGCs) are the first-line anti-inflammatory treatment of asthma and such treatment is associated with reduced peripheral blood T cell activation. Therefore we hypothesized that some of the examined parameters may be influenced by long-term IGCs therapy. Less is known about the effects of long-term IGCs therapy on humoral immunity in asthmatic children. To check if there are some differences in basal parameters assessing humoral immunity we evaluated serum IgA, IgM, and IgG levels in both groups.
Materials and Methods
The study group consisted of 47 children (aged 3–18 years) with allergic asthma. Ten had intermittent, 21 mild, 12 moderate, and 4 severe persistent asthma. All the children had attended the outpatient clinic at the Department of Pediatric Gastroenterology, Allergology and Developmental Disorders, Medical University of Silesia, Zabrze, for at least one year before recruitment into the study and had positive skin prick tests (SPTs) for one or more allergens (a SPT was regarded as positive when the mean diameter was at least 3 mm in the presence of negative diluent and positive histamine controls). All the children had significantly elevated total and relevant allergen-specific IgE levels. The diagnosis of asthma and the assessment of severity were done according to the GINA 2002 protocol [19,]. All the children had a history of recurrent episodes of airway obstruction. Children under six years of age underwent spirometric assessment and presented airway obstruction reversibility, as documented by positive bronchodilator responses of 12% FEV1 increase. Eight children younger than five years of age had parental histories of asthma and/or personal histories of atopic dermatitis (AD) and at least four episodes of wheezing and hospitalization for asthma in the year before enrollment in this study. Thirty-six children with mild to severe asthma were treated with regularly inhaled IGCs, but with a variable daily dose according to the asthma symptoms (at the time of evaluation the daily IGCs dose ranged from 100 to 1000 μg/day, mean daily dose: 264.5±41.94). The duration of IGCs treatment ranged from 2 months to 10 years. Fifteen asthmatic children had a history of AD and 23 had concomitant rhinitis symptoms. Detailed data of asthma duration and IGCs treatment were obtained from medical records and an interview questionnaire. The control group consisted of 50 healthy children (aged 3–17.5 years) with a negative history of allergic disease, normal levels of total serum IgE, and negative results of SPTs to a panel of inhaled allergens (dust mite, mixed grass or tree pollen, cat, dog; Allergopharma, Reinbek, Germany). The control children attended the outpatient pediatric clinic for non-immunological, non-inflammatory problems and required venous blood collection for routine laboratory tests.
The present study was approved by the Ethics Committee of the Medical University of Silesia in Katowice and written informed consent was obtained from the children’s parents.
Venous blood samples were obtained from each subject. The serum concentrations of total and specific IgE were measured using commercially available enzyme-linked immunosorbent assay kits (IU/ml, Allergopharma; detection threshold 1.0 IU/ml). Levels of total IgG, IgM, and IgA were determined by turbidimetry (COBA-S INTEGRA — 800).
Lymphocyte subsets in peripheral blood
Lymphocyte populations were analyzed by multiparameter flow cytometry with 3-color analyses. Phenotypes of the T cell populations were obtained with PerCP anti-CD3 antibody plus combinations of FITC- and PE-labeled antibodies specific to CD4, CD8, CD25, CD45RA, and CD45RO. The cells were stained with the appropriate isotope control antibodies and nonspecific staining was not observed. The antibodies used for flow cytometry were purchased from Becton Dickinson (San Jose, CA, USA). After staining, analysis was performed on a FACScan (Becton Dickinson) equipped with an argon laser tuned to 488 nm. The data were acquired and analyzed with Cell Quest software (Becton Dickinson). The proportion of lymphocytes stained with each monoclonal antibody was converted to the absolute count per microliter by multiplying the absolute number of lymphocytes per microliter derived from the complete blood count.
Statistical analysis was performed using the Statistica version 3.0 software package and data are presented as the mean value ±SE. The Mann-Whitney U-test was used for comparisons between groups. Correlations were calculated using Spearma’s rank correlation test. Values of p<0.05 were considered statistically significant.
Results
The demographic and background characteristics of the children studied are shown in Table 1. These two groups were similar with regard to age.
Comparison of eosinophil counts and serum IgA, IgM, IgG, and IgE levels between the children with asthma and controls
The percentage as well as the absolute count of peripheral blood eosinophils was significantly elevated in the asthmatic children compared with the control group (p<0.0005 and p<0.00005, respectively). Total serum IgE levels were significantly higher in the children with asthma (p<0.0005). Although serum IgA, IgM, and IgG levels in the children with asthma were within the normal range, IgM and IgG levels were significantly lower than in the controls (p<0.05 and p<0.01, respectively).
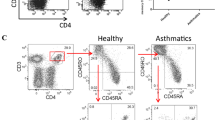
Expression of the naive/memory (CD45RA+/CD45RO+) markers on lymphocyte subsets in the children with asthma and the control group
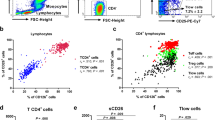
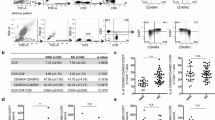
The mean percentages, counts, and SE for all the lymphocyte populations are shown in Table 2. Flow-cytometric analysis revealed no statistically significant differences in the percentages of CD3+, CD4+, CD8+ CD45RA+, CD4+CD45RA+, CD8+CD45RA+, CD45RO+, and CD8+CD45RO+ between the asthma group and healthy controls. The percentage of CD4+CD45RO+ was significantly lower in the asthmatic children (p<0.0002), but the absolute count did not differ between the groups. The CD8+CD45RO+ T cell count was significantly higher in the asthma group (p<0.025) than in the healthy children (Fig. 1).
The expression of naïve/memory markers in children with intermittent asthma (never IGCs treated) and with persistent asthma (IGCs treated) was similar. No significant correlation between dose of IGCs and expression of CD45RA/RO on T cells was found.
Percentages and absolute numbers of the CD4+ and CD8+ peripheral blood T-memory marker CD45RO in the study groups. The percentage of CD4+CD45RO+ T cells in children with asthma was significantly lower than in healthy children (p<0.0002), but the absolute numbers did not differ. The percentage of CD8+CD45RO+ T cells tended to be higher (p=0.1) and the number was significantly elevated in children with asthma compared with healthy children (p<0.025). The Mann-Whitney U-test was used to determine significant differences between the two groups.
Comparison of CD25 expression on CD3+ and CD4+ T cells between the children with asthma and the control group
The percentage, but not the absolute count, of CD3+CD25+ was significantly lower in the children with asthma than in the healthy children (p<0.05). Expression of CD25 on CD4+ T cells did not differ between the asthma and control group (Fig. 2). Duration of IGCs treatment inversely correlated with absolute count of circulating CD3+ and CD4+ T cells expressing the activation marker CD25 (R: −0.39, p=0.02 and R: −0.38, p=0.02, respectively). On the other hand, no correlation was found between the expression of CD25 on peripheral blood CD3+ and CD4+ T cells and duration of asthma.
Percentages and absolute numbers of CD3+ and CD4+ peripheral blood T cells expressing the activation marker CD25+ in the examined groups. The percentage of CD3+CD25+ T cells, but not the number, was significantly lower in children with asthma than in controls (p<0.05). Both the percentages and the numbers of CD4+CD25+ T cells were similar in the groups. The Mann-Whitney U-test was used to determine significant differences between the two groups.
Correlation between severity of asthma and immune parameters
Severity of asthma positively correlated with duration of asthma as well as serum IgE levels (R: 0.66, p=0.00001 and R: 0.57, p=0.00002), but inversely with spirometric parameters such as FEV1 and FEV1/FVC (R: −0.68, p=0.000002 and R: −0.53, p=0.0004, respectively, Fig. 3). No correlation was found between the severity of asthma and the age of the children. None of the examined peripheral blood T lymphocyte subsets correlated with the severity of asthma.
Discussion
Several studies have demonstrated increased numbers of CD4+ and CD8+ in bronchial biopsy specimens and bronchoalveolar larvage (BAL) fluid in subjects with asthma [2, 3]. Also, an imbalance of T cell subsets was documented in peripheral blood of asthmatics, especially after allergen challenge or during exacerbation of the disease [11, 15, 22]. In the present study we could not find differences in the percentages and numbers of CD3+, CD4+, and CD8+ T cells between children with asthma and non-atopic controls, which is in line with other reports [9]. However, our findings contrast with a recent study by Antunez et al. [1] showing a decrease in circulating CD8+ T cells in children with intermittent asthma.
Analyses of bronchial biopsy specimens and cells in BAL fluid from patients with asthma revealed elevated levels of activation marker CD25 (IL-2R) expression, even in the absence of an increase in T cells [2, 6, 13]. Peripheral blood CD4+, CD8+, and memory (CD45RO) T cells in atopic asthmatics also had higher expressions of IL-2R molecule compared with healthy controls [6, 13]. Surprisingly, children with asthma had a slightly lower number and significantly decreased proportion of CD3+CD25+ T cells and similar proportion and number of CD4+CD25+ T cells compared with healthy subjects. A possible explanation for this discrepancy is that most of the children in our study were regularly treated with IGCs, which inhibit T cell activation [10]. Indeed, there are many studies showing a reduction in the expression of the activation marker CD25 on T cells in airways as well as in peripheral blood in asthmatic patients after steroid treatment [6, 9, 17]. Of interest is that in our study a moderate negative correlation between the duration of IGCs treatment and the number of CD3+ and CD4+ T cells expressing the activation marker CD25 was observed.
It is known that among CD4+ T cells expressing CD25, a distinct subset with regulatory activity (CD4+CD25+ Treg) is present. Some recent reports have suggested the existence of defects in the number or function of CD4+CD25+ Treg in atopic individuals. Unfortunately, in this study we were not able to distinguish whether CD4+CD25+ are activated or regulatory T cells.
Imbalances in memory CD45RO+ T cells in the peripheral blood of patients with asthma and allergic disease have been reported, but results are inconsistent [1, 7, 12, 18]. We showed that the alteration in the peripheral blood memory T cell subpopulations in patients with asthma affects both the CD4+ and CD8+ T cell subsets. In this study the proportion of CD4+CD45RO+ cells was significantly lower in asthma patients than in controls; however, the absolute counts did not differ between the groups. The decrease in the percentage of CD4+CD4+ T cells may indicate that they were recruited from the peripheral blood to the site of inflammation, but taking into account the down-regulatory effect of glucocorticosteroids (GS) on CD45RO expression, our findings may also reflect the effect of long-term IGCs treatment. In previous studies, decreased expression of CD45RO in lungs [16] as well as on peripheral blood T cells in patients with asthma was associated with oral GC [6] or IGCs therapy [9]. On the other hand, in adult patients with asthma, despite of using a high dose of IGCs, the expression of CD45RO on CD4+ T cells was higher in severe asthma than in milder asthma and healthy controls [12]. In our study no correlation between severity of asthma and expression of the memory marker (CD45RO) on CD4+ T cells was found, although the percentage of CD4+CD45RO+ T cells correlated with the duration of asthma and the age of the examined patients. A lack of comparison of the expression of naïve/memory marker on circulating T cells before and after IGCs treatment in asthmatic children may be a drawback of this study. It is of note that the expressions of these markers in asthmatic children treated and never treated with IGCs were similar; moreover, no correlation between the dose of IGCs and the percentage/absolute count of CD45RO+ T cells was found.
In this study of children with asthma, the percentage of CD8+CD45RO+ T cells tended to be higher and the number of these cells was significantly elevated compared with non-atopic subjects. This result may suggest that besides CD4+, CD8+ memory T cells may also be implicated in the immunopathogenesis of asthma. Indeed, CD8+ T cells, which are capable of secreting Th2 cytokines, including IL-4, IL-5, and IL-13, have been described in asthmatic subjects and in animal models of asthma. It was shown that the number of CD8+ T cells in bronchial biopsies in asthma is associated with a decline in lung function [24]. Also, an increased cytokine production of sputum CD8+ T cells has been shown in patients with asthma that was related to disease severity [5]. Moreover, significantly greater expression of the activation marker CD25 by CD8+ T cells in peribronchial tissue in fatal asthma has been observed [20].
Some studies suggest that CD8+ T cells are relatively corticosteroid resistant [23]. It was shown that GS downregulate CD45RO only on CD4+, but not on CD8+ peripheral blood T cells [6, 9]. This may partially explain why a decrease in CD8+CD45RO+ T cells was not observed in our children.
In this study, similarly to other reports, we also showed that the severity of asthma correlated with total serum IgE levels and FEV1, but not with allergen-specific IgE and eosinophil levels [4]. Surprisingly, we found significantly decreased serum IgM and IgG concentrations in asthmatic children in comparison with healthy controls; nevertheless, all the children with asthma had serum IgA, IgM, and IgG within the normal range. We consider the possibility that long-term treatment with IGCs may be associated with this finding, since a recent study revealed an inhibitory effect of long-term low-dose GS therapy on humoral immunity [8].
In conclusion, significant differences can be detected in the memory compartment of CD4+ and CD8+ T cells in the peripheral blood of children with allergic asthma compared with healthy age-matched controls. This phenomenon is independent of the severity of the disease. Some differences in the expressions of activation markers on peripheral blood T cells may be related to long-term use of IGCs by children with asthma. The increase in CD8+CD45RO+ T cells in children with asthma may suggest an important role of this lymphocyte subpopulation in the pathogenesis of allergic diseases.
References
Antunez C., Torres M. J., Mayorga C., Corzo J. L., Jurado A., Santamaria-Babi L. F., Vera A. and Blanca M. (2006): Cytokine production, activation marker, and skin homing receptor in children with atopic dermatitis and bronchial asthma. Pediatr. Allergy Immunol., 17, 166–174.
Azzawi M., Bradley B., Jeffery P. K, Frew A. J., Wardlaw A. J., Knowles G., Assoufi B., Collins J. V., Durham S. and Kay A. B. (1990): Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am. Rev. Respir. Dis., 142, 1407–1413.
Bradley B. L., Azzawi M., Jacobson M., Assoufi B., Collins J. V., Irani A. M., Schwartz L. B., Durham S. R., Jeffery P. K. and Kay A. B. (1991): Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J. Allergy Clin. Immunol., 88, 661–674.
Carroll W. D., Lenney W., Child F., Strange R. C., Jones P. W., Whyte M. K., Primhak R. A. and Fryer A. A. (2006): Asthma severity and atopy: how clear is the relationship? Arch. Dis. Child., 91, 405–409.
Cho S. H., Stanciu L. A., Begishivili T., Bates P. J., Holgate S. T. and Johnston S. L. (2002): Peripheral blood CD4+ and CD8+ T cell type 1 and type 2 cytokine production in atopic asthmatic and normal subjects. Clin. Exp. Allergy, 32, 427–433.
Corrigan C. J., Haczku A., Gemou-Engesaeth V., Doi S., Kikuchi Y., Takatsu K., Durham S. R. and Kay A. B. (1993): CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5. Effect of glucocorticoid therapy. Am. Rev. Respir. Dis., 147, 540–547.
Dworzak M. N., Froschl G., Printz D., Fleischer C., Potschger U., Fritsch G., Gadner H., and Emminger W. (1999): Skin-associated lymphocytes in the peripheral blood of patients with atopic dermatitis: signs of subset expansion and stimulation. J. Allergy Clin. Immunol., 103, 901–906.
Fedor M. E. and Rubinstein A. (2006): Effects of long-term low-dose corticosteroid therapy on humoral immunity. Ann. Allergy Asthma Immunol., 97, 113–116.
Gemou-Engesaeth V., Fagerhol M. K., Toda M., Hamid Q., Halvorsen S., Groegaard J. B. and Corrigan C. J. (2002): Expression of activation markers and cytokine mRNA by peripheral blood CD4 and CD8 T cells in atopic and nonatopic childhood asthma: effect of inhaled glucocorticoid therapy. Pediatrics, 109, e24.
Georas S. N. (2004): Inhaled glucocorticoids, lymphocytes, and dendritic cells in asthma and obstructive ling diseases. Proc. Am. Thorac. Soc., 1, 215–221.
Gerblich A. A., Salik H. and Schuyler M. R. (1991): Dynamic T-cell changes in peripheral blood and bronchoalveolar lavage after antigen bronchoprovocation in asthmatics. Am. Rev. Respir. Dis., 143, 533–537.
Kurashima K., Fujimura M., Myou S., Ishiura Y., Onai N. and Matsushima K. (2006): Asthma severity is associated with an increase in both blood CXCR3+ and CCR4+ T cells. Respirology, 11, 152–157.
Lara-Marquez M. L., Moan M. J., Cartwright S., Listman J., Israel E., Perkins D. L., Christiani D. C. and Finn P. W. (2001): Atopic asthma: differential activation phenotypes among memory T helper cells. Clin. Exp. Allergy, 31, 1232–1241.
Larché M., Robinson D. S. and Kay A. B. (2003): The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol., 111, 450–463.
Lee S. Y., Kim S. J., Kwon S. S., Kim Y. K., Kim K. H., Moon H. S., Song J. S. and Park S. H. (2001): Distribution and cytokine production of CD4 and CD8 T-lymphocyte subsets in patients with acute asthma attacks. Ann. Allergy Asthma Immunol., 86, 659–664.
Liu M. C., Proud D., Lichtenstein L. M., Hubbard W. C., Bochner B. S., Stealey B. A., Breslin L., Xiao H., Freidhoff L. R., Schroeder J. T. and Schleimer R. P. (2001): Effects of prednisone on the cellular responses and release of cytokines and mediators after segmental allergen challenge of asthmatic subjects. J. Allergy Clin. Immunol., 108, 29–38.
Majori M., Piccoli M. L., Bertacco S., Cuomo A., Cantini L. and Pesci A. (1997): Inhaled beclomethasone dipropionate downregulates CD4 and CD8 T-lymphocyte activation in peripheral blood of patients with asthma. J. Allergy Clin. Immunol., 100, 379–382.
Matsuyama T., Urano K., Ohkido M., Ozawa H., Ohta A., Kaneko S., Yahata T., Takita C. and Nishimura T. (1999): The quantitative and qualitative defect of CD4+ CD45RO+ memory-type T cells are involved in the abnormality of TH1 immunity in atopic dermatitis patients. Clin. Exp. Allergy, 29, 687–694.
National Institutes of Health, National Heart, Lung, and Blood Institute (2002): Global Initiative for Asthma: global strategy for asthma management and prevention. Bethesda, MD, National Heart, Lung and Blood Institute/World Health Organization; NIH Publication No. 02-363659.
O’Sullivan S., Cormican L., Faul J. L., Ichinohe S., Johnston S. L., Burke C. M. and Poulter L. W. (2001): Activated, cytotoxic CD8(+) T lymphocytes contribute to the pathology of asthma death. Am. J. Respir. Crit. Care Med., 164, 560–564.
Seneviratne S. L., Jones L., King A. S., Black A., Powell S., McMichael A. J. and Ogg G. S. (2002): Allergen-specific CD8(+) T cells and atopic disease. J. Clin. Invest., 110, 1283–1291.
Shi H. Z., Sun J. J., Pan H. L., Lu J. Q., Zhang J. L. and Jiang J. D. (1999): Alterations of T-lymphocyte subsets, soluble IL-2 receptor, and IgE in peripheral blood of children with acute asthma attacks. J. Allergy Clin. Immunol., 103, 388–394.
Syed F., Bingham B., Johnson M., Markham A. F. and Morrison J. F. (1998): The CD4+ T lymphocyte is a site of steroid resistance in asthma. QJM, 91, 567–572.
van Rensen E. L., Sont J. K., Evertse C. E., Willems L. N, Mauad T., Hiemstra P. S., Sterk P. J. and AMPUL Study Group (2005): Bronchial CD8 cell infiltrate and lung function decline in asthma Am. J. Respir. Crit. Care Med., 172, 837–841.
Vignola A. M., Chanez P., Campbell A. M., Souques F., Lebel B., Enander I. and Bousquet J. (1998): Airway inflammation in mild intermittent and in persistent asthma. Am. J. Respir. Crit. Care Med., 157, 403–409.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Machura, E., Mazur, B., Pieniążek, W. et al. Expression of naive/memory (CD45RA/CD45RO) markers by peripheral blood CD4+ and CD8+ T cells in children with asthma. Arch. Immunol. Ther. Exp. 56, 55–62 (2008). https://doi.org/10.1007/s00005-008-0005-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-008-0005-6