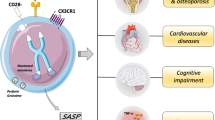
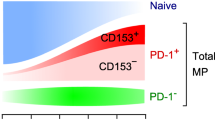
Abstract
Introduction
The incidence of rheumatoid arthritis (RA) and its complications are expected to increase with age. Remarkably, RA patients were identified features of accelerated aging, particularly in immunosenescence. As is known, T cells in RA patients readily differentiate into pro-inflammatory phenotypes that maintain chronic and persistent inflammatory changes in joints and many other organ systems. Recent evidence suggests that T cells are most sensitive to aging, and aged CD4+ T cells contribute to inflammaging, which plays a crucial role in accelerating the disease process. In recent years, the molecular mechanisms of T cell immunosenescence were beginning to be understood. Immune aging in RA T cells is associated with thymus insufficiency, metabolic abnormalities, shortened telomere length, and chronic energy stress. Therefore, we summarized the role and mechanism of T cell immunosenescence in RA.
Methods
A computer-based online search was performed using the PubMed database for published articles concerning T cells aging and rheumatoid arthritis.
Results
In this review, we assess the roles of CD4+ T cells in the center of inflammaging especially in RA and emphasize arthritogenic effector functions of senescent T cell; also we discuss the possible molecular mechanisms of senescent T cells and therapeutic targets to intervene T cells immunosenescence for improvement of RA.



Similar content being viewed by others
Abbreviations
- SASP:
-
Senescence-associated secretory phenotype
- TCR:
-
T cell receptor
- TEMRA:
-
Terminally differentiated effector memory T cell
- IGF-1:
-
Insulin-like growth factor-1
- FOXO:
-
Forkhead box O
- mTOR:
-
Mechanistic target of rapamycin
- MMPs:
-
Matrix metalloproteinases
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- Wnt:
-
Wingless/integrated
- LRP5/6:
-
LDL-receptor-related protein 5/6
- DDK-1:
-
Dickkopf-related protein 1
- ATM:
-
Ataxia telangiectasia-mutated
- DNA-PKcs:
-
DNA dependent protein kinase catalytic subunit
- PFKFB3:
-
Phosphofructokinase/fructose biphosphatase3
- G6PD:
-
Glucose-6-phosphate dehydrogenease
- DDR:
-
DNA damage response
- TCA:
-
Tricarboxylic acid cycle
- AMPK:
-
Adenosine 5’-monophosphate-activated protein kinase
- mTORC1:
-
Mechanistic target of rapamycin complex1
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome ten
- SIRT1:
-
NAD + -dependent deacetylase sirtuin-1
- Nrf2:
-
Nuclear factor erythroid2-related factor 2
- NHEJ:
-
Non-homologous end joining
- NMT1:
-
N-myristoyltransferase 1
- Drp1:
-
Dynamin-related protein 1
- PGC1α:
-
Peroxisome proliferator-activated receptor-γ coactivator-1α
References
Smolen, J.S., et al., Rheumatoid arthritis. Nature Reviews Disease Primers, 2018. 4(1).
Weyand CM, Zeisbrich M, Goronzy JJ. Metabolic signatures of T-cells and macrophages in rheumatoid arthritis. Curr Opin Immunol. 2017;46:112–20.
Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183–96.
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72.
McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet. 2017;389(10086):2328–37.
Dedmon LE. The genetics of rheumatoid arthritis. Rheumatol (Oxford). 2020;59(10):2661–70.
Amariuta T, et al. Advances in genetics toward identifying pathogenic cell states of rheumatoid arthritis. Immunol Rev. 2020;294(1):188–204.
Mellado M, et al. T cell migration in rheumatoid arthritis. Front Immunol. 2015;6:384.
Weyand CM, Goronzy JJ. Aging of the immune system mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(Suppl 5):S422–8.
Shirakawa K, Sano M. T cell immunosenescence in aging, obesity, and cardiovascular disease. Cells. 2021;10(9):2435.
Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol. 2021;22(6):687–98.
Moro-Garcia MA, et al. Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front Immunol. 2018;9:339.
Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10–9.
Goh J et al. Targeting the molecular and cellular pillars of human aging with exercise. FEBS J. 2021
Covre LP, et al. The role of senescent T cells in immunopathology. Aging Cell. 2020;19(12): e13272.
Bektas A, et al. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol. 2017;102(4):977–88.
Bauer ME. Accelerated immunosenescence in rheumatoid arthritis: impact on clinical progression. Immun Ageing. 2020;17:6.
Gonzalez-Osuna L, et al. Premature senescence of T-cells favors bone loss during osteolytic diseases a new concern in the osteoimmunology arena. Aging Dis. 2021;12(5):1150–61.
Sood A, Raji MA. Cognitive impairment in elderly patients with rheumatic disease and the effect of disease-modifying anti-rheumatic drugs. Clin Rheumatol. 2021;40(4):1221–31.
Koetz K, et al. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97(16):9203–8.
Fessler J, Angiari S. The role of T cell senescence in neurological diseases and its regulation by cellular metabolism. Front Immunol. 2021;12: 706434.
Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26(1):93–100.
Kunkl M, et al. T helper cells: the modulators of inflammation in multiple sclerosis. Cells. 2020;9(2):482.
Weyand CM, Goronzy JJ. Immunometabolism in the development of rheumatoid arthritis. Immunol Rev. 2020;294(1):177–87.
Petersen LE, et al. Characterization of senescence biomarkers in rheumatoid arthritis: relevance to disease progression. Clin Rheumatol. 2019;38(10):2909–15.
Trintinaglia L, et al. Features of immunosenescence in women newly diagnosed with breast cancer. Front Immunol. 2018;9:1651.
Plunkett FJ, et al. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178(12):7710–9.
Barbe-Tuana F, et al. The interplay between immunosenescence and age-related diseases. Semin Immunopathol. 2020;42(5):545–57.
Fessler J, et al. Premature senescence of T-cell subsets in axial spondyloarthritis. Ann Rheum Dis. 2016;75(4):748–54.
Luque-Campos N, et al. Mesenchymal stem cells improve rheumatoid arthritis progression by controlling memory T cell response. Front Immunol. 2019;10:798.
Minato N, Hattori M, Hamazaki Y. Physiology and pathology of T-cell aging. Int Immunol. 2020;32(4):223–31.
Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19(9):573–83.
Wang Y, et al. Germline genetic patterns underlying familial rheumatoid arthritis, systemic lupus erythematosus and primary Sjogren’s syndrome highlight T cell-initiated autoimmunity. Ann Rheum Dis. 2020;79(2):268–75.
Kroemer G, Zitvogel L. CD4(+) T cells at the center of inflammaging. Cell Metab. 2020;32(1):4–5.
Muscate F, Woestemeier A, Gagliani N. Functional heterogeneity of CD4(+) T cells in liver inflammation. Semin Immunopathol. 2021;43(4):549–61.
Morgan J, et al. Classical CD4 T cells as the cornerstone of antimycobacterial immunity. Immunol Rev. 2021;301(1):10–29.
van der Geest KSM, et al. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp Gerontol. 2014;60:190–6.
Chemin K, Gerstner C, Malmstrom V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation-lessons from rheumatoid arthritis. Front Immunol. 2019;10:353.
Ruterbusch M, et al. In vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol. 2020;38:705–25.
Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48(12):1379–86.
Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. 2018;95:77–99.
Bharath LP, et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 2020;32(1):44–55 (e6).
Sahmatova L, et al. Signs of innate immune activation and premature immunosenescence in psoriasis patients. Sci Rep. 2017;7(1):7553.
Ouyang X, et al. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cell Immunol. 2011;266(2):208–17.
Lucas C, Perdriger A, Ame P. Definition of B cell helper T cells in rheumatoid arthritis and their behavior during treatment. Semin Arthritis Rheum. 2020;50(5):867–72.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108.
Kerola AM, et al. Incidence, sociodemographic factors and treatment penetration of rheumatoid arthritis and psoriatic arthritis in Norway. Semin Arthritis Rheum. 2021;51(5):1081–8.
Chalan P, et al. Rheumatoid arthritis, immunosenescence and the hallmarks of aging. Curr Aging Sci. 2015;8(2):131–46.
Lenaers G, et al. Dysfunctional T cell mitochondria lead to premature aging. Trends Mol Med. 2020;26(9):799–800.
Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2(10):415–27.
Costenbader KH, et al. Immunosenescence and rheumatoid arthritis: does telomere shortening predict impending disease? Autoimmun Rev. 2011;10(9):569–73.
Lopez-Otin C, et al. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Chavez MD, Tse HM. Targeting mitochondrial-derived reactive oxygen species in T cell-mediated autoimmune diseases. Front Immunol. 2021;12: 703972.
Papadaki HA, et al. Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: evidence for a tumor necrosis factor alpha-mediated effect. Blood. 2002;99(5):1610–9.
Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity—catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5(5):225–34.
Li Z, Guo J, Bi L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed Pharmacother. 2020;130: 110542.
Patlán M, et al. Relative increase of Th17 phenotype in senescent CD4+CD28null T cells from peripheral blood of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2021;39(4):925–6.
Martin DE, et al. Targeting aging: lessons learned from immunometabolism and cellular senescence. Front Immunol. 2021;12: 714742.
Qiu J, et al. Metabolic control of autoimmunity and tissue inflammation in rheumatoid arthritis. Front Immunol. 2021;12: 652771.
ElTanbouly MA, Noelle RJ. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat Rev Immunol. 2021;21(4):257–67.
Darrigues J, van Meerwijk JPM, Romagnoli P. Age-dependent changes in regulatory t lymphocyte development and function: a mini-review. Gerontology. 2018;64(1):28–35.
Guo Z, et al. DCAF1 regulates treg senescence via the ROS axis during immunological aging. J Clin Invest. 2020;130(11):5893–908.
Wang T, et al. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 2015;74(6):1293–301.
Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58.
Robbins PD, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 2021;61:779–803.
Novais EJ, et al. Long-term treatment with senolytic drugs dasatinib and quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12(1):5213.
Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34(23–24):1565–76.
Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179.
Rathinam VAK, Chan FK. Inflammasome, inflammation, and tissue homeostasis. Trends Mol Med. 2018;24(3):304–18.
Boothby IC, Cohen JN, Rosenblum MD. Regulatory T cells in skin injury: at the crossroads of tolerance and tissue repair. Sci Immunol. 2020;5(47):eaaz9631.
Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–74.
Falconer J, et al. Review: synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2018;70(7):984–99.
Rao DA, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–4.
Pandya JM, et al. Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J Leukoc Biol. 2016;100(4):823–33.
Jeffery LE, et al. Decreased sensitivity to 1,25-dihydroxyvitamin D3 in T cells from the rheumatoid joint. J Autoimmun. 2018;88:50–60.
Weyand CM, Wu B, Goronzy JJ. The metabolic signature of T cells in rheumatoid arthritis. Curr Opin Rheumatol. 2020;32(2):159–67.
Salminen A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J Mol Med (Berl). 2021;99(1):1–20.
Chen Z, et al. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15(1):9–17.
Fessler J, et al. Novel senescent regulatory T-cell subset with impaired suppressive function in rheumatoid arthritis. Front Immunol. 2017;8:300.
Meyer A, et al. Kinase activity profiling reveals contribution of G-protein signaling modulator 2 deficiency to impaired regulatory T cell migration in rheumatoid arthritis. J Autoimmun. 2021;124:102726.
Corrado M, Pearce EL. Targeting memory T cell metabolism to improve immunity. J Clin Invest, 2022;132(1):e148546.
Fardellone P, et al. Bone Loss, Osteoporosis, and Fractures in Patients with Rheumatoid Arthritis: A Review. J Clin Med. 2020;9(10):3361.
Komatsu N et al. Plasma cells promote osteoclastogenesis and periarticular bone loss in autoimmune arthritis. J Clin Invest, 2021;131(6):e143060.
Zhao H, Lu A, He X. Roles of MicroRNAs in bone destruction of rheumatoid arthritis. Front Cell Dev Biol. 2020;8: 600867.
Cho SK, et al. Effectiveness of bazedoxifene in preventing glucocorticoid-induced bone loss in rheumatoid arthritis patients. Arthritis Res Ther. 2021;23(1):176.
Fessler J, et al. Senescent T-cells promote bone loss in rheumatoid arthritis. Front Immunol. 2018;9:95.
Wang G, et al. Cycloastragenol attenuates osteoclastogenesis and bone loss by targeting RANKL-induced Nrf2/Keap1/ARE, NF-kappaB, calcium, and NFATc1 pathways. Front Pharmacol. 2021;12: 810322.
Zerbini CAF, et al. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017;28(2):429–46.
Liu H, et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 2020;11(2):129.
Finzel S, et al. Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann Rheum Dis. 2019;78(9):1186–91.
Adam S, et al. JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci Transl Med. 2020;12(530):eaay4447.
Shim JH, Stavre Z, Gravallese EM. Bone loss in rheumatoid arthritis: basic mechanisms and clinical implications. Calcif Tissue Int. 2018;102(5):533–46.
Cici D, et al. Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int J Mol Sci. 2019;20(22):5552.
Szentpetery A, et al. Effects of targeted therapies on the bone in arthritides. Autoimmun Rev. 2017;16(3):313–20.
Najm A, et al. MicroRNA-17-5p reduces inflammation and bone erosions in mice with collagen-induced arthritis and directly targets the JAK/STAT pathway in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2020;72(12):2030–9.
Bhadricha H, et al. Increased frequency of Th17 cells and IL-17 levels are associated with low bone mineral density in postmenopausal women. Sci Rep. 2021;11(1):16155.
Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66(6):801–17.
Li Y, Goronzy JJ, Weyand CM. DNA damage, metabolism and aging in pro-inflammatory T cells: rheumatoid arthritis as a model system. Exp Gerontol. 2018;105:118–27.
Shao L. DNA damage response signals transduce stress from rheumatoid arthritis risk factors into T cell dysfunction. Front Immunol. 2018;9:3055.
Ummarino D. Rheumatoid arthritis: DNA repair links T-cell ageing to inflammation. Nat Rev Rheumatol. 2016;12(12):694.
Li Y, et al. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity. 2016;45(4):903–16.
Wiley CD, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23(2):303–14.
Li Y, et al. The DNA repair nuclease MRE11A functions as a mitochondrial protector and prevents T cell pyroptosis and tissue inflammation. Cell Metab. 2019;30(3):477–92 (e6).
Certo M, et al. Endothelial cell and T-cell crosstalk: targeting metabolism as a therapeutic approach in chronic inflammation. Br J Pharmacol. 2021;178(10):2041–59.
Weyand CM, Goronzy JJ. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(5):291–301.
Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci. 2015;1346(1):33–44.
Yang H, et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy. 2020;16(2):271–88.
Nnah IC, et al. TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy. 2019;15(1):151–64.
Reznick RM, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5(2):151–6.
Callender LA, et al. GATA3 induces mitochondrial biogenesis in primary human CD4(+) T cells during DNA damage. Nat Commun. 2021;12(1):3379.
Ye Z, et al. Regulation of miR-181a expression in T cell aging. Nat Commun. 2018;9(1):3060.
Xiong Y, et al. hPMSCs-derived exosomal miRNA-21 protects against aging-related oxidative damage of CD4(+) T cells by targeting the PTEN/PI3K-Nrf2 axis. Front Immunol. 2021;12: 780897.
Liu X, et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat Commun. 2018;9(1):249.
Chung JH. The role of DNA-PK in aging and energy metabolism. FEBS J. 2018;285(11):1959–72.
Gamal RM, et al. Telomere dysfunction-related serological markers and oxidative stress markers in rheumatoid arthritis patients: correlation with diseases activity. Clin Rheumatol. 2018;37(12):3239–46.
Cao D, et al. Disruption of telomere Integrity and DNA repair machineries by KML001 induces T cell senescence, apoptosis, and cellular dysfunctions. Front Immunol. 2019;10:1152.
Ji Y, et al. Topological DNA damage, telomere attrition and T cell senescence during chronic viral infections. Immun Ageing. 2019;16:12.
Wang Y, et al. Cytoplasmic DNA sensing by KU complex in aged CD4(+) T cell potentiates T cell activation and aging-related autoimmune inflammation. Immunity. 2021;54(4):632–47 (e9).
Yue X, et al. DNA-PKcs: a multi-faceted player in DNA damage response. Front Genet. 2020;11: 607428.
Cai WW, et al. Metabolic reprogramming as a key regulator in the pathogenesis of rheumatoid arthritis. Inflamm Res. 2020;69(11):1087–101.
Franco F, et al. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2(10):1001–12.
Yanes RE, et al. Metabolic reprogramming in memory CD4 T cell responses of old adults. Clin Immunol. 2019;207:58–67.
Peng H-Y, et al. Metabolic reprogramming and reactive oxygen species in T cell immunity. Front Immunol. 2021;12:652687.
McGuire PJ. Mitochondrial dysfunction and the aging immune system. Biol (Basel). 2019;8(2):26.
Pucino V, et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab. 2019;30(6):1055–74 (e8).
Yang Z, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8(331):331ra38.
Davalli P, et al. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:3565127.
Lavin MF, et al. ATM-dependent phosphorylation of all three members of the MRN complex: from sensor to adaptor. Biomolecules. 2015;5(4):2877–902.
Shen Y, et al. Metabolic control of the scaffold protein TKS5 in tissue-invasive, proinflammatory T cells. Nat Immunol. 2017;18(9):1025–34.
Myers DR, Wheeler B, Roose JP. mTOR and other effector kinase signals that impact T cell function and activity. Immunol Rev. 2019;291(1):134–53.
Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313.
Zeng H, Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol. 2013;25(3):347–55.
Lamming DW, Bar-Peled L. Lysosome: the metabolic signaling hub. Traffic. 2019;20(1):27–38.
Kundu-Raychaudhuri S, Abria C, Raychaudhuri SP. IL-9, a local growth factor for synovial T cells in inflammatory arthritis. Cytokine. 2016;79:45–51.
Pegoretti V, et al. Selective modulation of TNF-TNFRs signaling: insights for multiple sclerosis treatment. Front Immunol. 2018;9:925.
Su YJ, Wang PW, Weng SW. The role of mitochondria in immune-cell-mediated tissue regeneration and ageing. Int J Mol Sci. 2021;22(5):2668.
Correia-Melo C, et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35(7):724–42.
Callender LA, et al. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell. 2020;19(2): e13067.
Schroth J, Henson SM. Mitochondrial dysfunction accelerates ageing. Immunometabolism. 2020;2(4): e200035.
Clayton SA, et al. Mitochondria as key players in the pathogenesis and treatment of rheumatoid arthritis. Front Immunol. 2021;12: 673916.
Jiang Y, et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission-fusion dynamics and mitophagy. Redox Biol. 2022;52: 102304.
Moro L. Mitochondrial dysfunction in aging and cancer. J Clin Med. 2019;8(11):1983.
Fakouri NB, et al. Toward understanding genomic instability, mitochondrial dysfunction and aging. FEBS J. 2019;286(6):1058–73.
Raz Y, et al. Activation-induced autophagy Is preserved in CD4+ T-cells in familial longevity. J Gerontol A Biol Sci Med Sci. 2017;72(9):1201–6.
Bektas A, et al. Age-associated changes in human CD4(+) T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging (Albany NY). 2019;11(21):9234–63.
Vaena S, et al. Aging-dependent mitochondrial dysfunction mediated by ceramide signaling inhibits antitumor T cell response. Cell Rep. 2021;35(5): 109076.
Wyman B, Perl A. Metabolic pathways mediate pathogenesis and offer targets for treatment in rheumatic diseases. Curr Opin Rheumatol. 2020;32(2):184–91.
Tan S, et al. Platelet factor 4 enhances CD4(+) T effector memory cell responses via Akt-PGC1alpha-TFAM signaling-mediated mitochondrial biogenesis. J Thromb Haemost. 2020;18(10):2685–700.
Diot A, Morten K, Poulton J. Mitophagy plays a central role in mitochondrial ageing. Mamm Genome. 2016;27(7–8):381–95.
Willinger T, Flavell RA. Canonical autophagy dependent on the class III phosphoinositide-3 kinase Vps34 is required for naive T-cell homeostasis. Proc Natl Acad Sci USA. 2012;109(22):8670–5.
Baixauli F, et al. Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab. 2015;22(3):485–98.
Wu D, Prives C. Relevance of the p53-MDM2 axis to aging. Cell Death Differ. 2018;25(1):169–79.
Blandino G, et al. Wild type- and mutant p53 proteins in mitochondrial dysfunction: emerging insights in cancer disease. Semin Cell Dev Biol. 2020;98:105–17.
Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014;15(1):1–19.
Kabekkodu SP, et al. Clustered miRNAs and their role in biological functions and diseases. Biol Rev Camb Philos Soc. 2018;93(4):1955–86.
Lu Q, et al. miRNAs as therapeutic targets in inflammatory disease. Trends Pharmacol Sci. 2019;40(11):853–65.
Kroesen BJ, et al. Immuno-miRs: critical regulators of T-cell development, function and ageing. Immunology. 2015;144(1):1–10.
Kim C, et al. miR-181a-regulated pathways in T-cell differentiation and aging. Immun Ageing. 2021;18(1):28.
Gustafson CE, et al. Functional pathways regulated by microRNA networks in CD8 T-cell aging. Aging Cell. 2019;18(1): e12879.
Hammaker D, Firestein GS. Epigenetics of inflammatory arthritis. Curr Opin Rheumatol. 2018;30(2):188–96.
Teteloshvili N, et al. Involvement of MicroRNAs in the aging-related decline of CD28 expression by human T cells. Front Immunol. 2018;9:1400.
Yang P, et al. MicroRNA let-7g-5p alleviates murine collagen-induced arthritis by inhibiting Th17 cell differentiation. Biochem Pharmacol. 2020;174: 113822.
Jin S, et al. Protectin DX restores Treg/Th17 cell balance in rheumatoid arthritis by inhibiting NLRP3 inflammasome via miR-20a. Cell Death Dis. 2021;12(3):280.
Cheng NL, et al. MicroRNA-125b modulates inflammatory chemokine CCL4 expression in immune cells and its reduction causes CCL4 increase with age. Aging Cell. 2015;14(2):200–8.
Evangelatos G, et al. MicroRNAs in rheumatoid arthritis: from pathogenesis to clinical impact. Autoimmun Rev. 2019;18(11): 102391.
Cunningham CC, et al. Serum miRNA signature in rheumatoid arthritis and “at-risk individuals.” Front Immunol. 2021;12: 633201.
Guo D, et al. Study of miRNA interactome in active rheumatoid arthritis patients reveals key pathogenic roles of dysbiosis in the infection-immune network. Rheumatol (Oxford). 2021;60(3):1512–22.
Hanlon P, et al. Frailty in rheumatoidrmdopen arthritis and its relationship with disease activity, hospitalisation and mortality: a longitudinal analysis of the scottish early rheumatoid arthritis cohort and UK Biobank. RMD Open. 2022;8(1):e002111.
Salaffi F et al. Inflammaging and frailty in immune-mediated rheumatic diseases: how to address and score the issue. Clin Rev Allergy Immunol. 2022;8(1):e002111.
Acknowledgements
The authors wish to acknowledge Anhui engineering technology research center of biochemical pharmaceutical for the support of experimental equipment.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81703529), 512 Talent Training Program of Bengbu Medical College (by51202203), the Major research project of Education Department of Anhui Province (KJ2021ZD0086), Scientific and Technological Collaboration Project of Bengbu and Bengbu Medical College (BYLK201819), The Innovation and Entrepreneurship Project Plan of National Undergraduate Support Project of China (202010367037, 202110367020).
Author information
Authors and Affiliations
Contributions
YG designed and conducted the literature review and drafted the manuscript. WC, YZ, YL, and JC conducted the literature review. FW designed and conceptualized the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: Jason J. McDougall.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Cai, W., Zhou, Y. et al. Immunosenescence of T cells: a key player in rheumatoid arthritis. Inflamm. Res. 71, 1449–1462 (2022). https://doi.org/10.1007/s00011-022-01649-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-022-01649-0