Abstract
Polydnavirus-encoded IκB-like proteins are similar to insect and mammalian IκB, and an immunosuppressive function in the host cells has been inferred to these proteins. Here we show that the expression of one of these IκB-like viral genes, the TnBVank1, in the Drosophila germline affects the localization of gurken, bicoid, and oskar mRNAs whose gene products are relevant for proper embryonic patterning. The altered localization of these mRNAs is suggestive of general defects in the intracellular, microtubule-based, trafficking routes. Analysis of microtubule motor proteins components such as the dynein heavy chain and the kinesin heavy chain revealed defects in the polarized microtubule network. Interestingly, the TnBVANK1 viral protein is uniformly distributed over the entire oocyte cortex, and appears to be anchored to the microtubule ends. Our data open up a very interesting issue on novel function(s) played by the ank gene family by interfering with cytoskeleton organization.







Similar content being viewed by others
References
Pennacchio F, Strand MR (2006) Evolution of developmental strategies in parasitic hymenoptera. Annu Rev Entomol 51:233–258
Webb BA, Strand MR (2005) The biology and genomics of polydnaviruses. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive molecular insect science, vol 6. Elsevier, San Diego, pp 323–360
Webb BA, Beckage NE, Hayakawa Y, Krell PJ, Lanzrein B, Stoltz DB, Strand MR, Summers MD (2000) Family Polydnaviridae. In: van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB et al (eds) Virus taxonomy: seventh report of the international committee on taxonomy of viruses. Academic Press, San Diego, pp 253–260
Deng L, Stoltz DB, Webb BA (2000) A gene encoding a polydnavirus structural polypeptide is not encapsidated. Virology 269:440–450
Bézier A, Annaheim M, Herbinière J, Wetterwald C, Gyapay G, Bernard-Samain S, Wincker P, Roditi I, Heller M, Belghazi M, Pfister-Wilhem R, Periquet G, Dupuy C, Huguet E, Volkoff AN, Lanzrein B, Drezen JM (2009) Polydnaviruses of braconid wasps derive from an ancestral nudivirus. Science 323:926–930
Bézier A, Herbinière J, Lanzrein B, Drezen JM (2009) Polydnavirus hidden face: the genes producing virus particles of parasitic wasps. J Invertebr Pathol 101:194–203
Espagne E, Dupuy C, Huguet E, Cattolico L, Provost B, Martins N, Poirié M, Periquet G, Drezen JM (2004) Genome sequence of a polydnavirus: insights into symbiotic virus evolution. Science 306:286–289
Silverman N, Maniatis T (2001) NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev 15:2321–2342
Kroemer JA, Webb BA (2005) IκB-related vankyrin genes in the Campoletis sonorensis ichnovirus: temporal and tissue-specific patterns of expression in parasitized Heliothis virescens lepidopteran hosts. J Virol 79:7617–7628
Thoetkiattikul H, Beck MH, Strand MR (2005) Inhibitor κB-like proteins from a polydnavirus inhibit NF-κB activation and suppress the insect immune response. Proc Natl Acad Sci USA 102:11426–11431
Falabella P, Varricchio P, Provost B, Espagne E, Ferrarese R, Grimaldi A, de Eguileor M, Fimiani G, Ursini MV, Malva C, Drezen JM, Pennacchio F (2007) Characterization of the IκB-like gene family in polydnaviruses associated with wasps belonging to different Braconid subfamilies. J Gen Virol 88:92–104
Lapointe R, Tanaka K, Barney WE, Whitfield JB, Banks JC, Béliveau C, Stoltz D, Webb BA, Cusson M (2007) Genomic and morphological features of a banchine polydnavirus: comparison with bracoviruses and ichnoviruses. J Virol 81:6491–6501
Tian SP, Zhang JH, Wang CZ (2007) Cloning and characterization of two Campoletis chlorideae ichnovirus vankyrin genes expressed in parasitized host Helicoverpa armigera. J Insect Physiol 53:699–707
Shi M, Chen YF, Huang F, Liu PC, Zhou XP, Chen XX (2008) Characterization of a novel gene encoding ankyrin repeat domain from Cotesia vestalis polydnavirus (CvBV). Virology 375:374–382
Spencer W, Kwon H, Crepieux P, Leclerc N, Lin R, Hiscott J (1999) Taxol selectively blocks microtubule dependent NF-κB activation by phorbol ester via inhibition of IκBα phosphorylation and degradation. Oncogene 18:495–505
Mikenberg I, Widera D, Kaus A, Kaltschmidt B, Kaltschmidt C (2007) Transcription factor NF-κB is transported to the nucleus via cytoplasmic dynein/dynactin motor complex in hippocampal neurons. PLoS ONE 2:e589
Shrum CK, DeFrancisco D, Meffert MK (2009) Stimulated nuclear translocation of NF-κB and shuttling differentially depend on dynein and the dynactin complex. Proc Natl Acad Sci USA 106:2647–2652
Rizki TM, Rizki RM (1994) Parasitoid-induced cellular immune deficiency in Drosophila. Ann N Y Acad Sci 712:178–194
Glatz RV, Asgari S, Schmidt O (2004) Evolution of polydnaviruses as insect immune suppressors. Trends Microbiol 12:545–554
Spradling AC (1993) Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A et al (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 1–70
Steinhauer J, Kalderon D (2006) Microtubule polarity and axis formation in the Drosophila oocyte. Dev Dyn 235:1455–1468
Becalska AN, Gavis ER (2009) Lighting up mRNA localization in Drosophila oogenesis. Development 136:2493–2503
Cha BJ, Koppetsch BS, Theurkauf WE (2001) In vivo analysis of Drosophila bicoid mRNA localization reveals a novel microtubule-dependent axis specification pathway. Cell 106:35–46
MacDougall N, Clark A, MacDougall E, Davis I (2003) Drosophila gurken (TGFalpha) mRNA localizes as particles that move within the oocyte in two dynein-dependent steps. Dev Cell 4:307–319
Zimyanin VL, Belaya K, Pecreaux J, Gilchrist MJ, Clark A, Davis I, St Johnston D (2008) In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell 134:843–853
Clark I, Giniger E, Ruohola-Baker H, Jan LY, Jan YN (1994) Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr Biol 4:289–300
Clark IE, Jan LY, Jan YN (1997) Reciprocal localization of Nod and kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development 124:461–470
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415
Rorth P (1998) Gal4 in the Drosophila female germline. Mech Dev 78:113–118
Rubin GM, Spradling AC (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218:348–353
Tautz D, Pfeifle C (1989) A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98:81–85
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Andrenacci D, Cernilogar FM, Taddel C, Rotoli D, Cavaliere V, Graziani F, Gargiulo G (2001) Specific domains drive VM32E protein distribution and integration in Drosophila eggshell layers. J Cell Sci 114:2819–2829
Prasad M, Jang AC, Starz-Gaiano M, Melani M, Montell DJ (2007) A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat Protoc 2:2467–2473
Van Doren M, Williamson AL, Lehmann R (1998) Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol 8:243–246
Neuman-Silberberg FS, Schüpbach T (1993) The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell 75:165–174
Neuman-Silberberg FS, Schüpbach T (1996) The Drosophila TGF-alpha-like protein Gurken: expression and cellular localization during Drosophila oogenesis. Mech Dev 59:105–113
Schüpbach T (1987) Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell 49:699–707
Wasserman JD, Freeman M (1998) An autoregulatory cascade of EGF receptor signaling patterns the Drosophila egg. Cell 95:355–364
Peri F, Bokel C, Roth S (1999) Local Gurken signaling and dynamic MAPK activation during Drosophila oogenesis. Mech Dev 81:75–88
Gonzalez-Reyes A, Elliott H, St Johnston D (1995) Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 375:654–658
Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T (1995) Cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell 81:967–978
Hawkins NC, Van Buskirk C, Grossniklaus U, Schüpbach T (1997) Post-transcriptional regulation of gurken by encore is required for axis determination in Drosophila. Development 124:4801–4810
Saunders C, Cohen RS (1999) The role of oocyte transcription, the 5′UTR, and translation repression and derepression in Drosophila gurken mRNA and protein localization. Mol Cell 3:43–54
Norvell A, Kelley RL, Wehr K, Schüpbach T (1999) Specific isoforms of squid, a Drosophila hnRNP, perform distinct roles in Gurken localization during oogenesis. Genes Dev 13:864–876
St Johnston D, Nüsslein-Volhard C (1992) The origin of pattern and polarity in the Drosophila embryo. Cell 68:201–219
Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nüsslein-Volhard C (1988) The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J 7:1749–1756
Ephrussi A, Dickinson LK, Lehmann R (1991) Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66:37–50
McGrail M, Hays TS (1997) The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development 124:2409–2419
Li M, McGrail M, Serr M, Hays TS (1994) Drosophila cytoplasmic dynein, a microtubule motor that is asymmetrically localized in the oocyte. J Cell Biol 126:1475–1494
Matthies HJ, Baskin RJ, Hawley RS (2001) Orphan kinesin NOD lacks motile properties but does possess a microtubule-stimulated ATPase activity. Mol Biol Cell 12:4000–4012
Cui W, Sproul LR, Gustafson SM, Matthies HJ, Gilbert SP, Hawley RS (2005) Drosophila Nod protein binds preferentially to the plus ends of microtubules and promotes microtubule polymerization in vitro. Mol Biol Cell 16:5400–5409
Shapiro RS, Anderson KV (2006) Drosophila Iκ2, a member of the IκB kinase family, is required for mRNA localization during oogenesis. Development 133:1467–1475
Abdu U, Bar D, Schüpbach T (2006) spn-F encodes a novel protein that affects oocyte patterning and bristle morphology in Drosophila. Development 133:1477–1484
Provost B, Varricchio P, Arana E, Espagne E, Falabella P, Huguet E, La Scaleia R, Cattolico L, Poirié M, Malva C, Olszewski JA, Pennacchio F, Drezen JM (2004) Bracoviruses contain a large multigene family coding for protein tyrosine phosphatases. J Virol 78:13090–13103
Fath-Goodin A, Kroemer JA, Webb BA (2009) The Campoletis sonorensis ichnovirus vankyrin protein P-vank-1 inhibits apoptosis in insect Sf9 cells. Insect Mol Biol 18:497–506
Siegrist SE, Doe CQ (2007) Microtubule-induced cortical cell polarity. Genes Dev 21:483–496
Affolter M, Weijer CJ (2005) Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell 9:19–34
Etienne-Manneville S (2006) In vitro assay of primary astrocyte migration as a tool to study Rho GTPase function in cell polarization. Methods Enzymol 406:565–578
Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR (2008) Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320:1651–1654
Acknowledgments
Special thanks go to Carla Malva who inspired this work with her really original and open-minded vision of science. We thank Trudi Schüpbach, Anne Ephrussi, Tom Hays, and Daniel St. Johnston for kindly providing us antibodies and fly strains. We also thank the Bloomington Stock Center for fly stocks and the Developmental Studies Hybridoma Bank for antibodies. We are grateful to Angela Algeri for the careful reading of the manuscript. Our work was supported by grants from the MIUR and University of Bologna (Prin 2006/2008, RFO 2006; 2007). SD had a PhD fellowship from the University of Bologna.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2010_273_MOESM1_ESM.jpg
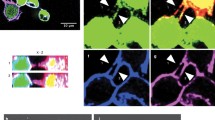
Distribution of TnBVANK1 protein. TnBVANK1 protein is localized in the cortical region of the oocyte, as shown by analyzing the oocyte just underneath its surface (A) where the protein shows a granular appearance, which is also detected in the cross section of the same stage 10B egg chamber (B). (JPG 1329 kb)
Rights and permissions
About this article
Cite this article
Duchi, S., Cavaliere, V., Fagnocchi, L. et al. The impact on microtubule network of a bracovirus IκB-like protein. Cell. Mol. Life Sci. 67, 1699–1712 (2010). https://doi.org/10.1007/s00018-010-0273-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0273-2