Abstract
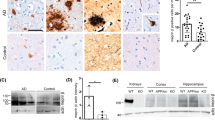
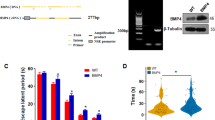
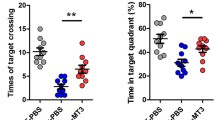
Among the dementias, Alzheimer’s disease (AD) is the most commonly diagnosed, but there are still no effective drugs available for its treatment. It has been suggested that metallothionein-3 (MT-3) could be somehow involved in the etiology of AD, and in fact very promising results have been found in in vitro studies, but the role of MT-3 in vivo needs further analysis. In this study, we analyzed the role of MT-3 in a mouse model of AD, Tg2576 mice, which overexpress human Amyloid Precursor Protein (hAPP) with the Swedish mutation. MT-3 deficiency partially rescued the APP-induced mortality of females, and mildly affected APP-induced changes in behavior assessed in the hole-board and plus-maze tests in a gender-dependent manner. Amyloid plaque burden and/or hAPP expression were decreased in the cortex and hippocampus of MT-3-deficient females. Interestingly, exogenously administered Zn7MT-3 increased soluble Aβ40 and Aβ42 and amyloid plaques and gliosis, particularly in the cortex, and changed several behavioral traits (increased deambulation and exploration and decreased anxiety). These results highlight that the control of the endogenous production and/or action of MT-3 could represent a powerful therapeutic target in AD.










Similar content being viewed by others
References
Adlard PA, Bush AI (2006) Metals and Alzheimer’s disease. J Alzheimers Dis 10:145–163
Amoureux MC, Van Gool D, Herrero MT, Dom R, Colpaert FC, Pauwels PJ (1997) Regulation of metallothionein-III (GIF) mRNA in the brain of patients with Alzheimer disease is not impaired. Mol Chem Neuropathol 32:101–121
Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A (1999) Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology 53:1992–1997
Angeletti B, Waldron KJ, Freeman KB, Bawagan H, Hussain I, Miller CC, Lau KF, Tennant ME, Dennison C, Robinson NJ, Dingwall C (2005) BACE1 cytoplasmic domain interacts with the copper chaperone for superoxide dismutase-1 and binds copper. J Biol Chem 280:17930–17937
Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D (2001) Behavioral assessment of Alzheimer’s transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol 20:737–744
Aschner M (1997) Astrocyte metallothioneins (MTs) and their neuroprotective role. Ann N Y Acad Sci 825:334–347
Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA (2005) Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 62:685–691
Bayer TA, Schäfer S, Simons A, Kemmling A, Kamer T, Tepest R, Eckert A, Schüssel K, Eikenberg O, Sturchler-Pierrat C, Abramowski D, Staufenbiel M, Multhaup G (2003) Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA 100:14187–14192
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW (2004) Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA 101:2173–2178
Borg J, Chereul E (2008) Differential MRI patterns of brain atrophy in double or single transgenic mice for APP and/or SOD. J Neurosci Res 86:3275–3284
Bruinink A, Faller P, Sidler C, Bogumil R, Vašák M (1998) Growth inhibitory factor and zinc affect neural cell cultures in a tissue specific manner. Chem Biol Interact 115:167–174
Bush AI, Tanzi RE (2008) Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 5:421–432
Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC (2001) Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol 158:1173–1177
Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK (1997) Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6:1951–1959
Carrasco J, Adlard P, Cotman C, Quintana A, Penkowa M, Xu F, Van Nostrand WE, Hidalgo J (2006) Metallothionein-I and -III expression in animal models of Alzheimer disease. Neuroscience 143:911–922
Carrasco J, Giralt M, Molinero A, Penkowa M, Moos T, Hidalgo J (1999) Metallothionein (MT)-III: generation of polyclonal antibodies, comparison with MT-I + II in the freeze lesioned rat brain and in a bioassay with astrocytes, and analysis of Alzheimer’s disease brains. J Neurotrauma 16:1115–1129
Carrasco J, Penkowa M, Giralt M, Camats J, Molinero A, Campbell IL, Palmiter RD, Hidalgo J (2003) Role of metallothionein-III following central nervous system damage. Neurobiol Dis 13:22–36
Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27:13357–13365
Ceballos D, Lago N, Verdu E, Penkowa M, Carrasco J, Navarro X, Palmiter RD, Hidalgo J (2003) Role of metallothioneins in peripheral nerve function and regeneration. Cell Mol Life Sci 60:1209–1216
Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM (2007) Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis 28:76–82
Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ (2002) Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res 70:462–473
Cole TB, Robbins CA, Wenzel HJ, Schwartzkroin PA, Palmiter RD (2000) Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res 39:153–169
Cyr DG, Dufresne J, Pillet S, Alfieri TJ, Hermo L (2001) Expression and regulation of metallothioneins in the rat epididymis. J Androl 22:124–135
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2:271–276
Chung RS, Howells C, Eaton ED, Shabala L, Zovo K, Palumaa P, Sillard R, Woodhouse A, Bennett WR, Ray S, Vickers JC, West AK (2010) The native copper- and zinc-binding protein metallothionein blocks copper-mediated Abeta aggregation and toxicity in rat cortical neurons. PLoS ONE 5:e12030
Chung RS, Vickers JC, Chuah MI, Eckhardt BL, West AK (2002) Metallothionein-III inhibits initial neurite formation in developing neurons as well as postinjury, regenerative neurite sprouting. Exp Neurol 178:1–12
De Strooper B (2010) Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 90:465–494
Dickstein DL, Biron KE, Ujiie M, Pfeifer CG, Jeffries AR, Jefferies WA (2006) Abeta peptide immunization restores blood-brain barrier integrity in Alzheimer disease. FASEB J 20:426–433
Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR (2003) Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 42:2768–2773
Durand J, Meloni G, Talmard C, Vašák M, Faller P (2010) Zinc release of Zn7-metallothionein-3 induces fibrillar type amyloid-β aggregates. Metallomics 2:741–744
El Ghazi I, Martin BL, Armitage IM (2006) Metallothionein-3 is a component of a multiprotein complex in the mouse brain. Exp Biol Med (Maywood) 231:1500–1506
El Ghazi I, Martin BL, Armitage IM (2010) New proteins found interacting with brain metallothionein-3 are linked to secretion. Int J Alzheimers Dis 2011:208634
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13:432–438
Erickson JC, Hollopeter G, Thomas SA, Froelick GJ, Palmiter RD (1997) Disruption of the metallothionein-III gene in mice: analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J Neurosci 17:1271–1281
Erickson JC, Masters BA, Kelly EJ, Brinster RL, Palmiter RD (1995) Expression of human metallothionein-III in transgenic mice. Neurochem Int 27:35–41
Erickson JC, Sewell AK, Jensen LT, Winge DR, Palmiter RD (1994) Enhanced neurotrophic activity in Alzheimer’s disease cortex is not associated with down-regulation of metallothionein-III (GIF). Brain Res 649:297–304
Faller P, Hasler DW, Zerbe O, Klauser S, Winge DR, Vašák M (1999) Evidence for a dynamic structure of human neuronal growth inhibitory factor and for major rearrangements of its metal- thiolate clusters. Biochemistry 38:10158–10167
Fernandes C, González MI, Wilson CA, File SE (1999) Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav 64:731–738
Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC (1994) Interleukin-6 secretion from human astrocytoma cells induced by substance P. J Neuroimmunol 51:101–108
Guglielmotto M, Giliberto L, Tamagno E, Tabaton M (2010) Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front Aging Neurosci 2:3
Hasler DW, Jensen LT, Zerbe O, Winge DR, Vašák M (2000) Effect of the two conserved prolines of human growth inhibitory factor (metallothionein-3) on its biological activity and structure fluctuation: comparison with a mutant protein. Biochemistry 39:14567–14575
Heneka MT, O’Banion MK, Terwel D, Kummer MP (2010) Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm 117:919–947
Hidalgo J, Penkowa M, Espejo C, Martínez-Cáceres EM, Carrasco J, Quintana A, Molinero A, Florit S, Giralt M, Ortega-Aznar A (2006) Expression of metallothionein-I, -II, and -III in Alzheimer disease and animal models of neuroinflammation. Exp Biol Med (Maywood) 231:1450–1458
Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4:97–100
Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D (1999) Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet 29:177–185
Hozumi I, Uchida Y, Watabe K, Sakamoto T, Inuzuka T (2006) Growth inhibitory factor (GIF) can protect from brain damage due to stab wounds in rat brain. Neurosci Lett 395:220–223
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Hua H, Münter L, Harmeier A, Georgiev O, Multhaup G, Schaffner W (2011) Toxicity of Alzheimer’s disease-associated Aβ peptide is ameliorated in a Drosophila model by tight control of zinc and copper availability. Biol Chem 392:919–926
Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA (1999) SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2:157–161
Iguchi K, Morihara N, Usui S, Hayama M, Sugimura Y, Hirano K (2011) Castration- and aging-induced changes in the expression of zinc transporter and metallothionein in rat prostate. J Androl 32:144–150
Irie Y, Keung WM (2001) Metallothionein-III antagonizes the neurotoxic and neurotrophic effects of amyloid beta peptides. Biochem Biophys Res Commun 282:416–420
Irie Y, Keung WM (2003) Anti-amyloid beta activity of metallothionein-III is different from its neuronal growth inhibitory activity: structure-activity studies. Brain Res 960:228–234
Ittner LM, Götz J (2011) Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12:65–72
King DL, Arendash GW (2002) Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav 75:627–642
King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ (1999) Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer’s disease. Behav Brain Res 103:145–162
Knipp M, Meloni G, Roschitzki B, Vašák M (2005) Zn7metallothionein-3 and the synaptic vesicle cycle: interaction of metallothionein-3 with the small GTPase Rab3A. Biochemistry 44:3159–3165
Koumura A, Kakefuda K, Honda A, Ito Y, Tsuruma K, Shimazawa M, Uchida Y, Hozumi I, Satoh M, Inuzuka T, Hara H (2009) Metallothionein-3 deficient mice exhibit abnormalities of psychological behaviors. Neurosci Lett 467:11–14
Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C (2005) Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol 167:527–543
Lahti DW, Hoekman JD, Tokheim AM, Martin BL, Armitage IM (2005) Identification of mouse brain proteins associated with isoform 3 of metallothionein. Protein Sci 14:1151–1157
Lalonde R, Dumont M, Staufenbiel M, Sturchler-Pierrat C, Strazielle C (2002) Spatial learning, exploration, anxiety, and motor coordination in female APP23 transgenic mice with the Swedish mutation. Brain Res 956:36–44
Lalonde R, Lewis TL, Strazielle C, Kim H, Fukuchi K (2003) Transgenic mice expressing the betaAPP695SWE mutation: effects on exploratory activity, anxiety, and motor coordination. Brain Res 977:38–45
Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY (2002) Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci USA 99:7705–7710
Lee JY, Mook-Jung I, Koh JY (1999) Histochemically reactive zinc in plaques of the Swedish mutant beta-amyloid precursor protein transgenic mice. J Neurosci 19:RC10:1–5
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA, McKeel D, Morris JC, Hulette CM, Schmechel D, Reiman EM, Rogers J, Stephan DA (2008) Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol Genomics 33:240–256
Manso Y, Adlard PA, Carrasco J, Vašák M, Hidalgo J (2011) Metallothionein and brain inflammation. J Biol Inorg Chem 16:1103–1113
Massaad CA, Washington TM, Pautler RG, Klann E (2009) Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106:13576–13581
Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, Brinster RL, Palmiter RD (1994) Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci 14:5844–5857
Maynard CJ, Cappai R, Volitakis I, Cherny RA, Masters CL, Li QX, Bush AI (2006) Gender and genetic background effects on brain metal levels in APP transgenic and normal mice: implications for Alzheimer beta-amyloid pathology. J Inorg Biochem 100:952–962
Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX (2002) Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem 277:44670–44676
Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L (2009) Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci Res 29:1977–1986
Meloni G, Knipp M, Vašák M (2005) Detection of neuronal growth inhibitory factor (metallothionein-3) in polyacrylamide gels and by Western blot analysis. J Biochem Biophys Methods 64:76–81
Meloni G, Sonois V, Delaine T, Guilloreau L, Gillet A, Teissié J, Faller P, Vasák M (2008) Metal swap between Zn7-metallothionein-3 and amyloid-beta-Cu protects against amyloid-beta toxicity. Nat Chem Biol 4:366–372
Meloni G, Vašák M (2011) Redox activity of α-synuclein-Cu is silenced by Zn7-metallothionein-3. Free Radic Biol Med 50:1471–1479
Moffatt P, Séguin C (1998) Expression of the gene encoding metallothionein-3 in organs of the reproductive system. DNA Cell Biol 17:501–510
Montoliu C, Monfort P, Carrasco J, Palacios O, Capdevila M, Hidalgo J, Felipo V (2000) Metallothionein-III prevents glutamate and nitric oxide neurotoxicity in primary cultures of cerebellar neurons. J Neurochem 75:266–273
Necula M, Kayed R, Milton S, Glabe CG (2007) Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem 282:10311–10324
Palumaa P, Tammiste I, Kruusel K, Kangur L, Jornvall H, Sillard R (2005) Metal binding of metallothionein-3 versus metallothionein-2: lower affinity and higher plasticity. Biochim Biophys Acta 1747:205–211
Pedersen AO, Jacobsen J (1980) Reactivity of the thiol group in human and bovine albumin at pH 3–9, as measured by exchange with 2,2′-dithiodipyridine. Eur J Biochem 106:291–295
Pedersen JT, Hureau C, Hemmingsen L, Heegaard NH, Ostergaard J, Vašák M, Faller P (2012) Rapid exchange of metal between Zn(7)-metallothionein-3 and amyloid-β peptide promotes amyloid-related structural changes. Biochem 51:1697–1706
Penkowa M, Tió L, Giralt M, Quintana A, Molinero A, Atrian S, Vašák M, Hidalgo J (2006) Specificity and divergence in the neurobiological effects of different metallothioneins after brain injury. J Neurosci Res 83:974–984
Phinney AL, Drisaldi B, Schmidt SD, Lugowski S, Coronado V, Liang Y, Horne P, Yang J, Sekoulidis J, Coomaraswamy J, Chishti MA, Cox DW, Mathews PM, Nixon RA, Carlson GA, St George-Hyslop P, Westaway D (2003) In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci USA 100:14193–14198
Pirev E, Ince Y, Sies H, Kröncke KD (2010) Heat shock but not cold shock leads to disturbed intracellular zinc homeostasis. J Cell Physiol 223:103–109
Pugh PL, Richardson JC, Bate ST, Upton N, Sunter D (2007) Non-cognitive behaviours in an APP/PS1 transgenic model of Alzheimer’s disease. Behav Brain Res 178:18–28
Puttaparthi K, Gitomer WL, Krishnan U, Son M, Rajendran B, Elliott JL (2002) Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J Neurosci 22:8790–8796
Romero-Isart N, Jensen LT, Zerbe O, Winge DR, Vašák M (2002) Engineering of metallothionein-3 neuroinhibitory activity into the inactive isoform metallothionein-1. J Biol Chem 277:37023–37028
Rosario ER, Carroll J, Pike CJ (2010) Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res 1359:281–290
Schäfer S, Pajonk FG, Multhaup G, Bayer TA (2007) Copper and clioquinol treatment in young APP transgenic and wild-type mice: effects on life expectancy, body weight, and metal-ion levels. J Mol Med (Berl) 85:405–413
Sewell AK, Jensen LT, Erickson JC, Palmiter RD, Winge DR (1995) Bioactivity of metallothionein-3 correlates with its novel beta domain sequence rather than metal binding properties. Biochemistry 34:4740–4747
Sinforiani E, Citterio A, Zucchella C, Bono G, Corbetta S, Merlo P, Mauri M (2010) Impact of gender differences on the outcome of Alzheimer’s disease. Dement Geriatr Cogn Disord 30:147–154
Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ (2000) Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res 852:274–278
Sultana R, Butterfield DA (2010) Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis 19:341–353
Toda T, Noda Y, Ito G, Maeda M, Shimizu T (2011) Presenilin-2 mutation causes early amyloid accumulation and memory impairment in a transgenic mouse model of Alzheimer’s disease. J Biomed Biotechnol 2011:617974
Tõugu V, Karafin A, Zovo K, Chung RS, Howells C, West AK, Palumaa P (2009) Zn(II)- and Cu(II)-induced non-fibrillar aggregates of amyloid-beta (1–42) peptide are transformed to amyloid fibrils, both spontaneously and under the influence of metal chelators. J Neurochem 110:1784–1795
Tsuji S, Kobayashi H, Uchida Y, Ihara Y, Miyatake T (1992) Molecular cloning of human growth inhibitory factor cDNA and its down-regulation in Alzheimer’s disease. EMBO J 11:4843–4850
Twine NA, Janitz K, Wilkins MR, Janitz M (2011) Whole transcriptome sequencing reveals gene expression and splicing differences in brain regions affected by Alzheimer’s disease. PLoS ONE 6:e16266
Uchida Y, Gomi F, Masumizu T, Miura Y (2002) Growth inhibitory factor prevents neurite extension and death of cortical neurons caused by high oxygen exposure through hydroxyl radical scavenging. J Biol Chem 277:32353–32359
Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M (1991) The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 7:337–347
Vašák M (1991) Metal removal and substitution in vertebrate and invertebrate metallothioneins. Methods Enzymol, 205:452–458
Vašák M, Meloni G (2011) Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem 16:1067–1078
Vest RS, Pike CJ (2012) Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav (in press). doi:10.1016/j.yhbeh.2012.04.006
Wang G, Zhang Y, Chen B, Cheng J (2003) Preliminary studies on Alzheimer’s disease using cDNA microarrays. Mech Ageing Dev 124:115–124
West AK, Hidalgo J, Eddins D, Levin ED, Aschner M (2008) Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology 29:489–503
West AK, Leung JY, Chung RS (2011) Neuroprotection and regeneration by extracellular metallothionein via lipoprotein-receptor-related proteins. J Biol Inorg Chem, 16:1115–1122
Yu WH, Lukiw WJ, Bergeron C, Niznik HB, Fraser PE (2001) Metallothionein III is reduced in Alzheimer’s disease. Brain Res 894:37–45
Acknowledgments
The authors are grateful for grants from the Ministerio de Ciencia e Innovación y Cofinanciada por el Fondo Europeo de Desarrollo Regional (FEDER), SAF2002-01268, SAF2005-00671, SAF2008-00435, and SAF2011-23272 (J.H.). Y.M. acknowledges her Ph.D. fellowship (AP2005-0588). P.A. is supported by the National Health and Medical Research Council of Australia, The Australian Research Council, The Alzheimer’s Association (USA), and the Joan and Peter Clemenger Trust. A.B. is a paid consultant and shareholder of Prana Biotechnology Ltd, and a paid consultant of Adenoa Inc, and a shareholder of Brighton Biotech Inc. P.A is a paid consultant and shareholder of Prana Biotechnology Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manso, Y., Carrasco, J., Comes, G. et al. Characterization of the role of metallothionein-3 in an animal model of Alzheimer’s disease. Cell. Mol. Life Sci. 69, 3683–3700 (2012). https://doi.org/10.1007/s00018-012-1047-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1047-9