Abstract
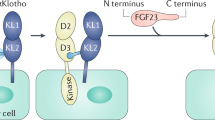
Following the serendipitous discovery of the ageing suppressor, αKlotho (αKl), several decades ago, a growing body of evidence has defined a pivotal role for its various forms in multiple aspects of vertebrate physiology and pathology. The transmembrane form of αKl serves as a co-receptor for the osteocyte-derived mineral regulator, fibroblast growth factor (FGF)23, principally in the renal tubules. However, compelling data also suggest that circulating soluble forms of αKl, derived from the same source, may have independent homeostatic functions either as a hormone, glycan-cleaving enzyme or lectin. Chronic kidney disease (CKD) is of particular interest as disruption of the FGF23–αKl axis is an early and common feature of disease manifesting in markedly deficient αKl expression, but FGF23 excess. Here we critically discuss recent findings in αKl biology that conflict with the view that soluble αKl has substantive functions independent of FGF23 signalling. Although the issue of whether soluble αKl can act without FGF23 has yet to be resolved, we explore the potential significance of these contrary findings in the context of CKD and highlight how this endocrine pathway represents a promising target for novel anti-ageing therapeutics.



Similar content being viewed by others
References
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51. https://doi.org/10.1038/36285
Tan SJ, Smith ER, Hewitson TD, Holt SG, Toussaint ND (2014) The importance of klotho in phosphate metabolism and kidney disease. Nephrology (Carlton) 19:439–449. https://doi.org/10.1111/nep.12268
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774. https://doi.org/10.1038/nature05315
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y (1998) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242:626–630
Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565:143–147. https://doi.org/10.1016/j.febslet.2004.03.090
Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M (2005) Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280:38029–38034. https://doi.org/10.1074/jbc.m509039200
Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T (2006) Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun 339:827–832. https://doi.org/10.1016/j.bbrc.2005.11.094
Kuro-o M (2008) Klotho as a regulator of oxidative stress and senescence. Biol Chem 389:233–241. https://doi.org/10.1515/bc.2008.028
Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S (2011) Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes 60:1907–1916. https://doi.org/10.2337/db10-1262
Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, Wang S, Xiao T, Xu X, He T, Xia X, Wang J, Zhao J (2015) Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol 26:2434–2446. https://doi.org/10.1681/asn.2014060543
Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M (2011) Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286:8655–8665. https://doi.org/10.1074/jbc.m110.174037
Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N (2012) Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol 303:F1641–F1651. https://doi.org/10.1152/ajprenal.00460.2012
Zhou L, Li Y, Zhou D, Tan RJ, Liu Y (2013) Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol 24:771–785. https://doi.org/10.1681/asn.2012080865
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833. https://doi.org/10.1126/science.1112766
Ullah M, Sun Z (2018) Klotho deficiency accelerates stem cells aging by impairing telomerase activity. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/gly261
Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, Hsia CC, Moe OW (2014) alpha-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol 307:L566–L575. https://doi.org/10.1152/ajplung.00306.2013
Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, Hu MC (2014) Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 85:855–870. https://doi.org/10.1038/ki.2013.489
Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, Takemura Y, Ohishi M, Katsuya T, Rakugi H (2009) Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 35:341–346. https://doi.org/10.1007/s12020-009-9181-3
Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, Oguro R, Yamamoto-Hanasaki H, Tatara Y, Takeya Y, Rakugi H (2011) Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int 11:510–516. https://doi.org/10.1111/j.1447-0594.2011.00699.x
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317:803–806. https://doi.org/10.1126/science.1143578
Kuro-o M, Hanaoka K, Hiroi Y, Noguchi T, Fujimori Y, Takewaki S, Hayasaka M, Katoh H, Miyagishi A, Nagai R et al (1995) Salt-sensitive hypertension in transgenic mice overexpressing Na(+)-proton exchanger. Circ Res 76:148–153
Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M (2005) Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126:1274–1283. https://doi.org/10.1016/j.mad.2005.07.007
Chen TH, Kuro OM, Chen CH, Sue YM, Chen YC, Wu HH, Cheng CY (2013) The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol 698:67–73. https://doi.org/10.1016/j.ejphar.2012.09.032
Smith ER (2018) Untangling the thread of life spun by αKlotho. J Mol Med 96:857–859. https://doi.org/10.1007/s00109-018-1671-4
Itoh N, Ornitz DM (2004) Evolution of the Fgf and Fgfr gene families. Trends Genet 20:563–569. https://doi.org/10.1016/j.tig.2004.08.007
Itoh N, Ohta H, Konishi M (2015) Endocrine FGFs: evolution, physiology, pathophysiology, and pharmacotherapy. Front Endocrinol (Lausanne) 6:154. https://doi.org/10.3389/fendo.2015.00154
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435. https://doi.org/10.1359/jbmr.0301264
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Investig 113:561–568. https://doi.org/10.1172/jci19081
Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6:744–759. https://doi.org/10.1002/emmm.201303716
Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG (2014) FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J 33:229–246. https://doi.org/10.1002/embj.201284188
Goetz R, Mohammadi M (2013) Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol 14:166–180. https://doi.org/10.1038/nrm3528
Mohammadi M, Olsen SK, Ibrahimi OA (2005) Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 16:107–137. https://doi.org/10.1016/j.cytogfr.2005.01.008
Ornitz DM, Itoh N (2015) The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol 4:215–266. https://doi.org/10.1002/wdev.176
Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE (2005) Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146:4647–4656. https://doi.org/10.1210/en.2005-0670
Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M (2007) Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27:3417–3428. https://doi.org/10.1128/mcb.02249-06
Kuro-O M, Moe OW (2017) FGF23-alphaKlotho as a paradigm for a kidney-bone network. Bone 100:4–18. https://doi.org/10.1016/j.bone.2016.11.013
Turan K, Ata P (2011) Effects of intra- and extracellular factors on anti-aging klotho gene expression. Genet Mol Res 10:2009–2023. https://doi.org/10.4238/vol10-3gmr1261
Xu Y, Sun Z (2015) Molecular basis of Klotho: from gene to function in aging. Endocr Rev 36:174–193. https://doi.org/10.1210/er.2013-1079
Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, Kerr Whitfield G (2011) Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun 414:557–562. https://doi.org/10.1016/j.bbrc.2011.09.117
Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N (2008) Klotho is a target gene of PPAR-gamma. Kidney Int 74:732–739. https://doi.org/10.1038/ki.2008.244
Tang R, Zhou QL, Ao X, Peng WS, Veeraragoo P, Tang TF (2011) Fosinopril and losartan regulate klotho gene and nicotinamide adenine dinucleotide phosphate oxidase expression in kidneys of spontaneously hypertensive rats. Kidney Blood Press Res 34:350–357. https://doi.org/10.1159/000326806
de Borst MH, Vervloet MG, ter Wee PM, Navis G (2011) Cross talk between the renin–angiotensin–aldosterone system and vitamin D-FGF-23–klotho in chronic kidney disease. J Am Soc Nephrol 22:1603–1609. https://doi.org/10.1681/asn.2010121251
Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD (2012) A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One 7:e44161. https://doi.org/10.1371/journal.pone.0044161
Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB (2011) The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol 22:1315–1325. https://doi.org/10.1681/asn.2010101073
Hiyama A, Arai F, Sakai D, Yokoyama K, Mochida J (2012) The effects of oxygen tension and antiaging factor Klotho on Wnt signaling in nucleus pulposus cells. Arthritis Res Ther 14:R105. https://doi.org/10.1186/ar3830
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123. https://doi.org/10.1074/jbc.c500457200
Goetz R, Ohnishi M, Ding X, Kurosu H, Wang L, Akiyoshi J, Ma J, Gai W, Sidis Y, Pitteloud N, Kuro OM, Razzaque MS, Mohammadi M (2012) Klotho coreceptors inhibit signaling by paracrine fibroblast growth factor 8 subfamily ligands. Mol Cell Biol 32:1944–1954. https://doi.org/10.1128/mcb.06603-11
Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M (2018) alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553:461–466. https://doi.org/10.1038/nature25451
Goetz R, Ohnishi M, Kir S, Kurosu H, Wang L, Pastor J, Ma J, Gai W, Kuro-o M, Razzaque MS, Mohammadi M (2012) Conversion of a paracrine fibroblast growth factor into an endocrine fibroblast growth factor. J Biol Chem 287:29134–29146. https://doi.org/10.1074/jbc.m112.342980
Smith ER, Tan SJ, Holt SG, Hewitson TD (2017) FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci Rep 7:3345. https://doi.org/10.1038/s41598-017-02709-w
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M (2011) FGF23 induces left ventricular hypertrophy. J Clin Investig 121:4393–4408. https://doi.org/10.1172/jci46122
Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstadt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C (2015) Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 22:1020–1032. https://doi.org/10.1016/j.cmet.2015.09.002
Silswal N, Touchberry CD, Daniel DR, McCarthy DL, Zhang S, Andresen J, Stubbs JR, Wacker MJ (2014) FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab 307:E426–E436. https://doi.org/10.1152/ajpendo.00264.2014
Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, Di Marco GS, Brand M, Wolf M, Faul C (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90:985–996. https://doi.org/10.1016/j.kint.2016.05.019
Murali SK, Roschger P, Zeitz U, Klaushofer K, Andrukhova O, Erben RG (2016) FGF23 regulates bone mineralization in a 1,25(OH)2 D3 and Klotho-independent manner. J Bone Miner Res 31:129–142. https://doi.org/10.1002/jbmr.2606
Murali SK, Andrukhova O, Clinkenbeard EL, White KE, Erben RG (2016) Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp mice. PLoS Biol 14:e1002427. https://doi.org/10.1371/journal.pbio.1002427
Olauson H, Lindberg K, Amin R, Sato T, Jia T, Goetz R, Mohammadi M, Andersson G, Lanske B, Larsson TE (2013) Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLoS Genet 9:e1003975. https://doi.org/10.1371/journal.pgen.1003975
Hensel N, Schon A, Konen T, Lubben V, Forthmann B, Baron O, Grothe C, Leifheit-Nestler M, Claus P, Haffner D (2016) Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density. J Neurochem 137:756–769. https://doi.org/10.1111/jnc.13585
Yang K, Peretz-Soroka H, Wu J, Zhu L, Cui X, Zhang M, Rigatto C, Liu Y, Lin F (2017) Fibroblast growth factor 23 weakens chemotaxis of human blood neutrophils in microfluidic devices. Sci Rep 7:3100. https://doi.org/10.1038/s41598-017-03210-0
Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstadt HJ, Meersch M, Unruh M, Zarbock A (2016) FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Investig 126:962–974. https://doi.org/10.1172/jci83470
Krick S, Grabner A, Baumlin N, Yanucil C, Helton S, Grosche A, Sailland J, Geraghty P, Viera L, Russell DW, Wells JM, Xu X, Gaggar A, Barnes J, King GD, Campos M, Faul C, Salathe M (2018) Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J. https://doi.org/10.1183/13993003.00236-2018
Barnes JW, Duncan D, Helton S, Hutcheson S, Kurundkar D, Logsdon NJ, Locy M, Garth J, Denson R, Farver C, Vo HT, King G, Kentrup D, Faul C, Kulkarni T, De Andrade JA, Yu Z, Matalon S, Thannickal VJ, Krick S (2019) Role of fibroblast growth factor 23 and klotho cross talk in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 317:L141–L154. https://doi.org/10.1152/ajplung.00246.2018
Smith ER, Holt SG, Hewitson TD (2017) FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-beta autoinduction. Int J Biochem Cell Biol 92:63–78. https://doi.org/10.1016/j.biocel.2017.09.009
Vainikka S, Joukov V, Wennstrom S, Bergman M, Pelicci PG, Alitalo K (1994) Signal transduction by fibroblast growth factor receptor-4 (FGFR-4). Comparison with FGFR-1. J Biol Chem 269:18320–18326
Wang JK, Gao G, Goldfarb M (1994) Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol Cell Biol 14:181–188
Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K (2004) Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29:91–99
Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao LL, Hiemstra TF, Zehnder D (2015) alpha-Klotho expression in human tissues. J Clin Endocrinol Metab 100:E1308–E1318. https://doi.org/10.1210/jc.2015-1800
van Loon EP, Pulskens WP, van der Hagen EA, Lavrijsen M, Vervloet MG, van Goor H, Bindels RJ, Hoenderop JG (2015) Shedding of klotho by ADAMs in the kidney. Am J Physiol Renal Physiol 309:F359–F368. https://doi.org/10.1152/ajprenal.00240.2014
Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C (2009) Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583:3221–3224. https://doi.org/10.1016/j.febslet.2009.09.009
Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR (2007) Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104:19796–19801. https://doi.org/10.1073/pnas.0709805104
Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, Abraham CR (2014) Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 53:5579–5587. https://doi.org/10.1021/bi500409n
Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE (2014) The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25:2169–2175. https://doi.org/10.1681/asn.2013111209
Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, Kuro-o M, Moe OW (2016) Renal production, uptake, and handling of circulating alphaKlotho. J Am Soc Nephrol 27:79–90. https://doi.org/10.1681/asn.2014101030
Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y (1998) Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424:6–10
Chateau MT, Araiz C, Descamps S, Galas S (2010) Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging (Albany NY) 2:567–581. https://doi.org/10.18632/aging.100195
Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW, Sidhu SS (2015) The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 30:223–233. https://doi.org/10.1093/ndt/gfu291
Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, Vervloet MG, Consortium N (2013) Laboratory aspects of circulating alpha-Klotho. Nephrol Dial Transplant 28:2283–2287. https://doi.org/10.1093/ndt/gft236
Mencke R, Harms G, Moser J, van Meurs M, Diepstra A, Leuvenink HG, Hillebrands JL (2017) Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight. https://doi.org/10.1172/jci.insight.94375
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310:490–493. https://doi.org/10.1126/science.1114245
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105:9805–9810. https://doi.org/10.1073/pnas.0803223105
Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24:3438–3450. https://doi.org/10.1096/fj.10-154765
Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y (2007) alpha-Klotho as a regulator of calcium homeostasis. Science 316:1615–1618. https://doi.org/10.1126/science.1135901
Hum JM, O’Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, Bhaskaran M, Johnson RL, Wilson JM, Smith RC, White KE (2017) Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble klotho. J Am Soc Nephrol 28:1162–1174. https://doi.org/10.1681/asn.2015111266
Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG (2011) Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int 89:140–150. https://doi.org/10.1007/s00223-011-9501-5
Wlodawer A, Minor W, Dauter Z, Jaskolski M (2013) Protein crystallography for aspiring crystallographers or how to avoid pitfalls and traps in macromolecular structure determination. FEBS J 280:5705–5736. https://doi.org/10.1111/febs.12495
Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M (2010) Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23–FGFR–Klotho complex formation. Proc Natl Acad Sci USA 107:407–412. https://doi.org/10.1073/pnas.0902006107
Tribolo S, Berrin JG, Kroon PA, Czjzek M, Juge N (2007) The crystal structure of human cytosolic beta-glucosidase unravels the substrate aglycone specificity of a family 1 glycoside hydrolase. J Mol Biol 370:964–975. https://doi.org/10.1016/j.jmb.2007.05.034
Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y (2002) Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta 1576:341–345
Matern H, Boermans H, Lottspeich F, Matern S (2001) Molecular cloning and expression of human bile acid beta-glucosidase. J Biol Chem 276:37929–37933. https://doi.org/10.1074/jbc.m104290200
Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL (2009) Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol 76:38–46. https://doi.org/10.1124/mol.109.055780
Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y (2004) Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem 279:9777–9784. https://doi.org/10.1074/jbc.m312392200
Xie J, Yoon J, An SW, Kuro-o M, Huang CL (2015) Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26:1150–1160. https://doi.org/10.1681/asn.2014040325
Liu F, Wu S, Ren H, Gu J (2011) Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 13:254–262. https://doi.org/10.1038/ncb2167
Wright JD, An SW, Xie J, Lim C, Huang CL (2019) Soluble klotho regulates TRPC6 calcium signaling via lipid rafts, independent of the FGFR–FGF23 pathway. Faseb J. https://doi.org/10.1096/fj.201900321r
Wright JD, An SW, Xie J, Yoon J, Nischan N, Kohler JJ, Oliver N, Lim C, Huang CL (2017) Modeled structural basis for the recognition of alpha2–3-sialyllactose by soluble Klotho. Faseb J 31:3574–3586. https://doi.org/10.1096/fj.201700043r
Dalton G, An SW, Al-Juboori SI, Nischan N, Yoon J, Dobrinskikh E, Hilgemann DW, Xie J, Luby-Phelps K, Kohler JJ, Birnbaumer L, Huang CL (2017) Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci USA 114:752–757. https://doi.org/10.1073/pnas.1620301114
Sugano Y, Lardelli M (2011) Identification and expression analysis of the zebrafish orthologue of Klotho. Dev Genes Evol 221:179–186. https://doi.org/10.1007/s00427-011-0367-3
Ohnishi M, Razzaque MS (2010) Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 24:3562–3571. https://doi.org/10.1096/fj.09-152488
Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD (2007) Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol 18:2116–2124. https://doi.org/10.1681/asn.2006121385
Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ (2007) A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact 7:318–319
Ramnitz MS, Gourh P, Goldbach-Mansky R, Wodajo F, Ichikawa S, Econs MJ, White KE, Molinolo A, Chen MY, Heller T, Del Rivero J, Seo-Mayer P, Arabshahi B, Jackson MB, Hatab S, McCarthy E, Guthrie LC, Brillante BA, Gafni RI, Collins MT (2016) Phenotypic and genotypic characterization and treatment of a cohort with familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome. J Bone Miner Res 31:1845–1854. https://doi.org/10.1002/jbmr.2870
Erben RG (2018) α-Klotho’s effects on mineral homeostasis are fibroblast growth factor-23 dependent. Curr Opin Nephrol Hypertens 27:229–235. https://doi.org/10.1097/mnh.0000000000000415
Andrukhova O, Bayer J, Schuler C, Zeitz U, Murali SK, Ada S, Alvarez-Pez JM, Smorodchenko A, Erben RG (2017) Klotho lacks an FGF23-independent role in mineral homeostasis. J Bone Miner Res 32:2049–2061. https://doi.org/10.1002/jbmr.3195
Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro OM, Hill JA, Moe OW (2015) Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 26:1290–1302. https://doi.org/10.1681/asn.2014050465
Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T (2008) Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene 27:7094–7105. https://doi.org/10.1038/onc.2008.292
Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, Sonomura K, Adachi Y, Shibuya M, Shirayama T, Tanda S, Hatta T, Sasaki S, Mori Y, Matsubara H (2010) Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci USA 107:19308–19313. https://doi.org/10.1073/pnas.1008544107
Kuro OM (2018) The Klotho proteins in health and disease. Nat Rev Nephrol. https://doi.org/10.1038/s41581-018-0078-3
Guan X, Nie L, He T, Yang K, Xiao T, Wang S, Huang Y, Zhang J, Wang J, Sharma K, Liu Y, Zhao J (2014) Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J Pathol 234:560–572. https://doi.org/10.1002/path.4420
Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B, Wolf I (2011) KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res 17:4254–4266. https://doi.org/10.1158/1078-0432.ccr-10-2749
Richter B, Faul C (2018) FGF23 actions on target tissues-with and without klotho. Front Endocrinol (Lausanne) 9:189. https://doi.org/10.3389/fendo.2018.00189
Hu MC, Kuro-o M, Moe OW (2013) Klotho and chronic kidney disease. Contrib Nephrol 180:47–63. https://doi.org/10.1159/000346778
Moe OW (2012) Fibroblast growth factor 23: friend or foe in uremia? J Clin Investig 122:2354–2356. https://doi.org/10.1172/jci64184
Wolf M (2012) Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82:737–747. https://doi.org/10.1038/ki.2012.176
Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:1370–1378. https://doi.org/10.1038/ki.2011.47
Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22:124–136. https://doi.org/10.1681/asn.2009121311
Neyra JA, Hu MC (2016) alphaKlotho and chronic kidney disease. Vitam Horm 101:257–310. https://doi.org/10.1016/bs.vh.2016.02.007
Azuma M, Koyama D, Kikuchi J, Yoshizawa H, Thasinas D, Shiizaki K, Kuro-o M, Furukawa Y, Kusano E (2012) Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J 26:4264–4274. https://doi.org/10.1096/fj.12-211631
Pavik I, Jaeger P, Kistler AD, Poster D, Krauer F, Cavelti-Weder C, Rentsch KM, Wuthrich RP, Serra AL (2011) Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int 79:234–240. https://doi.org/10.1038/ki.2010.375
Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro OM, Moe OW (2017) Recombinant alpha-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 91:1104–1114. https://doi.org/10.1016/j.kint.2016.10.034
Tan SJ, Crosthwaite A, Langsford D, Obeysekere V, Ierino FL, Roberts MA, Hughes PD, Hewitson TD, Dwyer KM, Toussaint ND (2017) Mineral adaptations following kidney transplantation. Transpl Int 30:463–473. https://doi.org/10.1111/tri.12925
Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE (2012) Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Investig 122:4710–4715. https://doi.org/10.1172/jci64986
Knothe Tate ML, Niederer P, Knothe U (1998) In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22:107–117
Knothe Tate ML (2003) “Whither flows the fluid in bone?” An osteocyte’s perspective. J Biomech 36:1409–1424
Smith ER (2014) The use of fibroblast growth factor 23 testing in patients with kidney disease. Clin J Am Soc Nephrol 9:1283–1303. https://doi.org/10.2215/cjn.10941013
Smith ER, McMahon LP, Holt SG (2014) Fibroblast growth factor 23. Ann Clin Biochem 51:203–227. https://doi.org/10.1177/0004563213510708
Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M (2014) Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25:349–360. https://doi.org/10.1681/asn.2013050465
Hao H, Li X, Li Q, Lin H, Chen Z, Xie J, Xuan W, Liao W, Bin J, Huang X, Kitakaze M, Liao Y (2016) FGF23 promotes myocardial fibrosis in mice through activation of beta-catenin. Oncotarget 7:64649–64664. https://doi.org/10.18632/oncotarget.11623
Takashi Y, Kinoshita Y, Hori M, Ito N, Taguchi M, Fukumoto S (2017) Patients with FGF23-related hypophosphatemic rickets/osteomalacia do not present with left ventricular hypertrophy. Endocr Res 42:132–137. https://doi.org/10.1080/07435800.2016.1242604
Chesher D, Oddy M, Darbar U, Sayal P, Casey A, Ryan A, Sechi A, Simister C, Waters A, Wedatilake Y, Lachmann RH, Murphy E (2018) Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J Inherit Metab Dis 41:865–876. https://doi.org/10.1007/s10545-018-0147-6
Pastor-Arroyo EM, Gehring N, Krudewig C, Costantino S, Bettoni C, Knopfel T, Sabrautzki S, Lorenz-Depiereux B, Pastor J, Strom TM, Hrabe de Angelis M, Camici GG, Paneni F, Wagner CA, Rubio-Aliaga I (2018) The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int 94:49–59. https://doi.org/10.1016/j.kint.2018.02.017
Liu ES, Thoonen R, Petit E, Yu B, Buys ES, Scherrer-Crosbie M, Demay MB (2018) Increased circulating FGF23 does not lead to cardiac hypertrophy in the male Hyp mouse model of XLH. Endocrinology 159:2165–2172. https://doi.org/10.1210/en.2018-00174
Leifheit-Nestler M, Richter B, Basaran M, Nespor J, Vogt I, Alesutan I, Voelkl J, Lang F, Heineke J, Krick S, Haffner D (2018) Impact of altered mineral metabolism on pathological cardiac remodeling in elevated fibroblast growth factor 23. Front Endocrinol (Lausanne) 9:333. https://doi.org/10.3389/fendo.2018.00333
Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, Drueke TB, Herzog CA, London GM, Mahaffey KW, Wheeler DC, Stolina M, Dehmel B, Goodman WG, Floege J (2015) Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 132:27–39. https://doi.org/10.1161/circulationaha.114.013876
Pi M, Ye R, Han X, Armstrong B, Liu X, Chen Y, Sun Y, Quarles LD (2018) Cardiovascular interactions between fibroblast growth factor-23 and angiotensin II. Sci Rep 8:12398. https://doi.org/10.1038/s41598-018-30098-1
Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL (2012) Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3:1238. https://doi.org/10.1038/ncomms2240
Xu H, Hashem A, Witasp A, Mencke R, Goldsmith D, Barany P, Bruchfeld A, Wernerson A, Carrero JJ, Olauson H (2018) Fibroblast growth factor 23 is associated with fractional excretion of sodium in patients with chronic kidney disease. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy315
Akhabue E, Montag S, Reis JP, Pool LR, Mehta R, Yancy CW, Zhao L, Wolf M, Gutierrez OM, Carnethon MR, Isakova T (2018) FGF23 (fibroblast growth factor-23) and incident hypertension in young and middle-aged adults: the CARDIA study. Hypertension 72:70–76. https://doi.org/10.1161/hypertensionaha.118.11060
Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, Singh S, Wolf M, Hermann S, Stypmann J, Di Marco GS, Brand M, Wacker MJ, Faul C (2017) FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep 7:1993. https://doi.org/10.1038/s41598-017-02068-6
Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, Landray MJ, Moe SM, Yang J, Holland L, di Giuseppe R, Bouma-de Krijger A, Mihaylova B, Herrington WG (2018) Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: a meta-analysis. J Am Soc Nephrol 29:2015–2027. https://doi.org/10.1681/asn.2017121334
Matsui I, Oka T, Kusunoki Y, Mori D, Hashimoto N, Matsumoto A, Shimada K, Yamaguchi S, Kubota K, Yonemoto S, Higo T, Sakaguchi Y, Takabatake Y, Hamano T, Isaka Y (2018) Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int 94:60–71. https://doi.org/10.1016/j.kint.2018.02.018
Richter M, Lautze HJ, Walther T, Braun T, Kostin S, Kubin T (2015) The failing heart is a major source of circulating FGF23 via oncostatin M receptor activation. J Heart Lung Transplant 34:1211–1214. https://doi.org/10.1016/j.healun.2015.06.007
Insogna KL, Briot K, Imel EA, Kamenicky P, Ruppe MD, Portale AA, Weber T, Pitukcheewanont P, Cheong HI, Jan de Beur S, Imanishi Y, Ito N, Lachmann RH, Tanaka H, Perwad F, Zhang L, Chen CY, Theodore-Oklota C, Mealiffe M, San Martin J, Carpenter TO (2018) A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res 33:1383–1393. https://doi.org/10.1002/jbmr.3475
Carpenter TO, Whyte MP, Imel EA, Boot AM, Hogler W, Linglart A, Padidela R, Van’t Hoff W, Mao M, Chen CY, Skrinar A, Kakkis E, San Martin J, Portale AA (2018) Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 378:1987–1998. https://doi.org/10.1056/NEJMoa1714641
Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG (2012) FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Investig 122:2543–2553. https://doi.org/10.1172/jci61405
Zhang Q, Liu L, Lin W, Yin S, Duan A, Liu Z, Cao W (2017) Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int 91:144–156. https://doi.org/10.1016/j.kint.2016.07.040
Yin S, Zhang Q, Yang J, Lin W, Li Y, Chen F, Cao W (2017) TGFbeta-incurred epigenetic aberrations of miRNA and DNA methyltransferase suppress Klotho and potentiate renal fibrosis. Biochim Biophys Acta Mol Cell Res 1864:1207–1216. https://doi.org/10.1016/j.bbamcr.2017.03.002
Irifuku T, Doi S, Sasaki K, Doi T, Nakashima A, Ueno T, Yamada K, Arihiro K, Kohno N, Masaki T (2016) Inhibition of H3K9 histone methyltransferase G9a attenuates renal fibrosis and retains klotho expression. Kidney Int 89:147–157. https://doi.org/10.1038/ki.2015.291
Lin W, Li Y, Chen F, Yin S, Liu Z, Cao W (2017) Klotho preservation via histone deacetylase inhibition attenuates chronic kidney disease-associated bone injury in mice. Sci Rep 7:46195. https://doi.org/10.1038/srep46195
Lin W, Zhang Q, Liu L, Yin S, Liu Z, Cao W (2017) Klotho restoration via acetylation of peroxisome proliferation-activated receptor gamma reduces the progression of chronic kidney disease. Kidney Int 92:669–679. https://doi.org/10.1016/j.kint.2017.02.023
Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y (2003) Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17:2393–2403. https://doi.org/10.1210/me.2003-0048
Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM (2012) Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 82:1261–1270. https://doi.org/10.1038/ki.2012.322
Hsu SC, Huang SM, Chen A, Sun CY, Lin SH, Chen JS, Liu ST, Hsu YJ (2014) Resveratrol increases anti-aging Klotho gene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int J Biochem Cell Biol 53:361–371. https://doi.org/10.1016/j.biocel.2014.06.002
Kuwahara N, Sasaki S, Kobara M, Nakata T, Tatsumi T, Irie H, Narumiya H, Hatta T, Takeda K, Matsubara H, Hushiki S (2008) HMG-CoA reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int J Cardiol 123:84–90. https://doi.org/10.1016/j.ijcard.2007.02.029
Sugiura H, Yoshida T, Mitobe M, Shiohira S, Nitta K, Tsuchiya K (2010) Recombinant human erythropoietin mitigates reductions in renal klotho expression. Am J Nephrol 32:137–144. https://doi.org/10.1159/000315864
Tataranni T, Biondi G, Cariello M, Mangino M, Colucci G, Rutigliano M, Ditonno P, Schena FP, Gesualdo L, Grandaliano G (2011) Rapamycin-induced hypophosphatemia and insulin resistance are associated with mTORC2 activation and Klotho expression. Am J Transplant 11:1656–1664. https://doi.org/10.1111/j.1600-6143.2011.03590.x
Shin YJ, Luo K, Quan Y, Ko EJ, Chung BH, Lim SW, Yang CW (2019) Therapeutic challenge of minicircle vector encoding klotho in animal model. Am J Nephrol 49:413–424. https://doi.org/10.1159/000499863
Neyra JA, Hu MC (2017) Potential application of klotho in human chronic kidney disease. Bone 100:41–49. https://doi.org/10.1016/j.bone.2017.01.017
Mencke R, Olauson H, Hillebrands JL (2017) Effects of Klotho on fibrosis and cancer: a renal focus on mechanisms and therapeutic strategies. Adv Drug Deliv Rev 121:85–100. https://doi.org/10.1016/j.addr.2017.07.009
Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC (2016) alphaKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol 27:2331–2345. https://doi.org/10.1681/ASN.2015060613
Jin M, Lv P, Chen G, Wang P, Zuo Z, Ren L, Bi J, Yang CW, Mei X, Han D (2017) Klotho ameliorates cyclosporine A-induced nephropathy via PDLIM2/NF-kB p65 signaling pathway. Biochem Biophys Res Commun 486:451–457. https://doi.org/10.1016/j.bbrc.2017.03.061
Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y (2015) Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin–angiotensin system. Am J Pathol 185:3211–3223. https://doi.org/10.1016/j.ajpath.2015.08.004
Deng M, Luo Y, Li Y, Yang Q, Deng X, Wu P, Ma H (2015) Klotho gene delivery ameliorates renal hypertrophy and fibrosis in streptozotocin-induced diabetic rats by suppressing the Rho-associated coiled-coil kinase signaling pathway. Mol Med Rep 12:45–54. https://doi.org/10.3892/mmr.2015.3367
Liu X, Chen Y, McCoy CW, Zhao T, Quarles DL, Pi M, Bhattacharya SK, King G, Sun Y (2016) Differential regulatory role of soluble klothos on cardiac fibrogenesis in hypertension. Am J Hypertens 29:1140–1147. https://doi.org/10.1093/ajh/hpw062
Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H (2017) Klotho suppresses the renin–angiotensin system in adriamycin nephropathy. Nephrol Dial Transplant 32:791–800. https://doi.org/10.1093/ndt/gfw340
Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H (2018) Klotho ameliorates medullary fibrosis and pressure natriuresis in hypertensive rat kidneys. Hypertension 72:1151–1159. https://doi.org/10.1161/hypertensionaha.118.11176
Jou-Valencia D, Molema G, Popa E, Aslan A, van Dijk F, Mencke R, Hillebrands JL, Heeringa P, Hoenderop JG, Zijlstra JG, van Meurs M, Moser J (2018) Renal klotho is reduced in septic patients and pretreatment with recombinant klotho attenuates organ injury in lipopolysaccharide-challenged mice. Crit Care Med 46:e1196–e1203. https://doi.org/10.1097/ccm.0000000000003427
Takenaka T, Kobori H, Miyazaki T, Suzuki H, Nishiyama A, Ishii N, Yamashita M, Hayashi M (2019) Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol (Oxf) 225:e13190. https://doi.org/10.1111/apha.13190
Wu YL, Xie J, An SW, Oliver N, Barrezueta NX, Lin MH, Birnbaumer L, Huang CL (2017) Inhibition of TRPC6 channels ameliorates renal fibrosis and contributes to renal protection by soluble klotho. Kidney Int 91:830–841. https://doi.org/10.1016/j.kint.2016.09.039
Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW (2010) Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78:1240–1251. https://doi.org/10.1038/ki.2010.328
Song S, Gao P, Xiao H, Xu Y, Si LY (2013) Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS One 8:e82968. https://doi.org/10.1371/journal.pone.0082968
Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP (2008) A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA 105:3455–3460. https://doi.org/10.1073/pnas.0712361105
Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M (2000) Crystal structure of a ternary FGF–FGFR–heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell 6:743–750
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Holt SG, Smith ER (2016) Fetuin-A-containing calciprotein particles in mineral trafficking and vascular disease. Nephrol Dial Transplant 31:1583–1587. https://doi.org/10.1093/ndt/gfw048
Pasch A, Jahnen-Dechent W, Smith ER (2018) Phosphate, calcification in blood, and mineral stress: the physiologic blood mineral buffering system and its association with cardiovascular risk. Int J Nephrol 2018:9182078. https://doi.org/10.1155/2018/9182078
Acknowledgements
The authors were supported by a Grant-in-Aid (GIA-021-2017) from the RMH Home Lottery Research Fund. The figures in this review were partly generated using vector images freely available from Servier Medical Art (http://smart.servier.com) which is licensed under a Creative Commons Attribution 3.0 Unported License.
Author information
Authors and Affiliations
Contributions
ERS, TDH: manuscript drafting, revision and final approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no relevant conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Smith, E.R., Holt, S.G. & Hewitson, T.D. αKlotho–FGF23 interactions and their role in kidney disease: a molecular insight. Cell. Mol. Life Sci. 76, 4705–4724 (2019). https://doi.org/10.1007/s00018-019-03241-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03241-y