Abstract
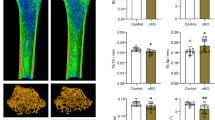
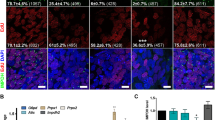
Mammalian cardiomyocytes (CMs) undergo maturation during postnatal heart development to meet the increased demands of growth. Here, we found that omentin-1, an adipokine, facilitates CM cell cycle arrest and metabolic maturation. Deletion of omentin-1 causes mouse heart enlargement and dysfunction in adulthood and CM maturation retardation in juveniles, including delayed cell cycle arrest and reduced fatty acid oxidation. Through RNA sequencing, molecular docking analysis, and proximity ligation assays, we found that omentin-1 regulates CM maturation by interacting directly with bone morphogenetic protein 7 (BMP7). Omentin-1 prevents BMP7 from binding to activin type II receptor B (ActRIIB), subsequently decreasing the downstream pathways mothers against DPP homolog 1 (SMAD1)/Yes-associated protein (YAP) and p38 mitogen-activated protein kinase (p38 MAPK). In addition, omentin-1 is required and sufficient for the maturation of human embryonic stem cell-derived CMs. Together, our findings reveal that omentin-1 is a pro-maturation factor for CMs that is essential for postnatal heart development and cardiac function maintenance.









Similar content being viewed by others
Data availability
The sequencing datasets involved in this work have been deposited in the NCBI database under project accession number PRJNA681365.
References
Zaffran S, Frasch M (2002) Early signals in cardiac development. Circ Res 91:457–469. https://doi.org/10.1161/01.res.0000034152.74523.a8
Guo Y, Pu WT (2020) Cardiomyocyte maturation: new phase in development. Circ Res 126:1086–1106. https://doi.org/10.1161/CIRCRESAHA.119.315862
Kannan S, Kwon C (2020) Regulation of cardiomyocyte maturation during critical perinatal window. J Physiol 598:2941–2956. https://doi.org/10.1113/JP276754
Sim CB, Phipson B, Ziemann M et al (2021) Sex-specific control of human heart maturation by the progesterone receptor. Circulation 143:1614–1628. https://doi.org/10.1161/CIRCULATIONAHA.120.051921
Puente BN, Kimura W, Muralidhar SA et al (2014) The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157:565–579. https://doi.org/10.1016/j.cell.2014.03.032
Li Y, Feng J, Song S et al (2020) gp130 controls cardiomyocyte proliferation and heart regeneration. Circulation 142:967–982. https://doi.org/10.1161/CIRCULATIONAHA.119.044484
Li R, Xiang C, Li Y, Nie Y (2023) Targeting immunoregulation for cardiac regeneration. J Mol Cell Cardiol 177:1–8. https://doi.org/10.1016/j.yjmcc.2023.02.003
Wang Y, Li Y, Feng J et al (2020) Mydgf promotes cardiomyocyte proliferation and neonatal heart regeneration. Theranostics 10:9100–9112. https://doi.org/10.7150/thno.44281
Guo Y, Cao Y, Jardin BD et al (2021) Sarcomeres regulate murine cardiomyocyte maturation through MRTF-SRF signaling. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2008861118
Fukuda R, Gunawan F, Ramadass R et al (2019) Mechanical forces regulate cardiomyocyte myofilament maturation via the VCL-SSH1-CFL axis. Dev Cell 51:62–77. https://doi.org/10.1016/j.devcel.2019.08.006
Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW 2nd (2015) Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 350:aad2459. https://doi.org/10.1126/science.aad2459
Kuppusamy KT, Jones DC, Sperber H et al (2015) Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci U S A 112:E2785-2794. https://doi.org/10.1073/pnas.1424042112
Lopaschuk GD, Jaswal JS (2010) Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol 56:130–140. https://doi.org/10.1097/FJC.0b013e3181e74a14
Wilsbacher L, McNally EM (2016) Genetics of cardiac developmental disorders: cardiomyocyte proliferation and growth and relevance to heart failure. Annu Rev Pathol 11:395–419. https://doi.org/10.1146/annurev-pathol-012615-044336
Ai S, Peng Y, Li C et al (2017) EED orchestration of heart maturation through interaction with HDACs is H3K27me3-independent. Elife. https://doi.org/10.7554/eLife.24570
Liang X, Sun Y, Ye M et al (2009) Targeted ablation of PINCH1 and PINCH2 from murine myocardium results in dilated cardiomyopathy and early postnatal lethality. Circulation 120:568–576. https://doi.org/10.1161/CIRCULATIONAHA.109.864686
Hu P, Liu J, Zhao J, Wilkins BJ, Lupino K, Wu H, Pei L (2018) Single-nucleus transcriptomic survey of cell diversity and functional maturation in postnatal mammalian hearts. Genes Dev 32:1344–1357. https://doi.org/10.1101/gad.316802.118
Liu C, Spinozzi S, Chen JY et al (2019) Nexilin Is a new component of junctional membrane complexes required for cardiac T-tubule formation. Circulation 140:55–66. https://doi.org/10.1161/CIRCULATIONAHA.119.039751
Metrich M, Bezdek Pomey A, Berthonneche C, Sarre A, Nemir M, Pedrazzini T (2015) Jagged1 intracellular domain-mediated inhibition of Notch1 signalling regulates cardiac homeostasis in the postnatal heart. Cardiovasc Res 108:74–86. https://doi.org/10.1093/cvr/cvv209
Parikh SS, Blackwell DJ, Gomez-Hurtado N et al (2017) Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res 121:1323–1330. https://doi.org/10.1161/CIRCRESAHA.117.311920
Sakamoto T, Matsuura TR, Wan S et al (2020) A critical role for estrogen-related receptor signaling in cardiac maturation. Circ Res 126:1685–1702. https://doi.org/10.1161/CIRCRESAHA.119.316100
Guo Y, Jardin BD, Zhou P et al (2018) Hierarchical and stage-specific regulation of murine cardiomyocyte maturation by serum response factor. Nat Commun 9:3837. https://doi.org/10.1038/s41467-018-06347-2
Mahmoud AI, Kocabas F, Muralidhar SA et al (2013) Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497:249–253. https://doi.org/10.1038/nature12054
Nguyen NUN, Canseco DC, Xiao F et al (2020) A calcineurin-Hoxb13 axis regulates growth mode of mammalian cardiomyocytes. Nature 582:271–276. https://doi.org/10.1038/s41586-020-2228-6
Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL (2012) Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J 26:397–408. https://doi.org/10.1096/fj.10-179895
Chattergoon NN, Louey S, Scanlan T, Lindgren I, Giraud GD, Thornburg KL (2019) Thyroid hormone receptor function in maturing ovine cardiomyocytes. J Physiol 597:2163–2176. https://doi.org/10.1113/JP276874
Bassat E, Mutlak YE, Genzelinakh A et al (2017) The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547:179–184. https://doi.org/10.1038/nature22978
Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma XL, Ouchi N (2017) Role of adipokines in cardiovascular disease. Circ J 81:920–928. https://doi.org/10.1253/circj.CJ-17-0458
Smekal A, Vaclavik J (2017) Adipokines and cardiovascular disease: A comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 161:31–40. https://doi.org/10.5507/bp.2017.002
Kuba K, Zhang L, Imai Y et al (2007) Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ Res 101:e32-42. https://doi.org/10.1161/CIRCRESAHA.107.158659
Liao Y, Takashima S, Maeda N et al (2005) Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res 67:705–713. https://doi.org/10.1016/j.cardiores.2005.04.018
Hall ME, Smith G, Hall JE, Stec DE (2012) Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in Cre-recombinase-mediated cardiotoxicity. Am J Physiol Regul Integr Comp Physiol 303:R1241-1250. https://doi.org/10.1152/ajpregu.00292.2012
McGaffin KR, Witham WG, Yester KA, Romano LC, O’Doherty RM, McTiernan CF, O’Donnell CP (2011) Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res 89:60–71. https://doi.org/10.1093/cvr/cvq288
Narumi T, Watanabe T, Kadowaki S et al (2014) Impact of serum omentin-1 levels on cardiac prognosis in patients with heart failure. Cardiovasc Diabetol 13:84. https://doi.org/10.1186/1475-2840-13-84
Huang Y, Lin Y, Zhang S et al (2016) Circulating Omentin-1 levels are decreased in dilated cardiomyopathy patients with overt heart failure. Dis Markers 2016:6762825. https://doi.org/10.1155/2016/6762825
Yildiz SS, Sahin I, Cetinkal G et al (2018) Usefulness of serum omentin-1 levels for the prediction of adverse cardiac events in patients with hypertrophic cardiomyopathy. Med Princ Pract 27:107–114. https://doi.org/10.1159/000487396
Matsuo K, Shibata R, Ohashi K et al (2015) Omentin functions to attenuate cardiac hypertrophic response. J Mol Cell Cardiol 79:195–202. https://doi.org/10.1016/j.yjmcc.2014.11.019
Kataoka Y, Shibata R, Ohashi K et al (2014) Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol 63:2722–2733. https://doi.org/10.1016/j.jacc.2014.03.032
Cardoso-Moreira M, Halbert J, Valloton D et al (2019) Gene expression across mammalian organ development. Nature 571:505–509. https://doi.org/10.1038/s41586-019-1338-5
Rao SS, Hu Y, Xie PL et al (2018) Omentin-1 prevents inflammation-induced osteoporosis by downregulating the pro-inflammatory cytokines. Bone Res 6:9. https://doi.org/10.1038/s41413-018-0012-0
Li J, Zhu D, Hu S, Nie Y (2022) CRISPR-CasRx knock-in mice for RNA degradation. Sci China Life Sci 65:2248–2256. https://doi.org/10.1007/s11427-021-2059-5
Li Y, Li H, Pei J, Hu S, Nie Y (2021) Transplantation of murine neonatal cardiac macrophage improves adult cardiac repair. Cell Mol Immunol 18:492–494. https://doi.org/10.1038/s41423-020-0371-5
Ackers-Johnson M, Li PY, Holmes AP, O’Brien SM, Pavlovic D, Foo RS (2016) A simplified, langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ Res 119:909–920. https://doi.org/10.1161/CIRCRESAHA.116.309202
Yue Z, Chen J, Lian H et al (2019) PDGFR-beta signaling regulates cardiomyocyte proliferation and myocardial regeneration. Cell Rep 28:966–978. https://doi.org/10.1016/j.celrep.2019.06.065
Li H, Liu C, Bao M, Liu W, Nie Y, Lian H, Hu S (2020) Optimized Langendorff perfusion system for cardiomyocyte isolation in adult mouse heart. J Cell Mol Med 24:14619–14625. https://doi.org/10.1111/jcmm.15773
Feng J, Li Y, Nie Y (2022) Methods of mouse cardiomyocyte isolation from postnatal heart. J Mol Cell Cardiol 168:35–43. https://doi.org/10.1016/j.yjmcc.2022.04.007
Karakikes I, Senyei GD, Hansen J et al (2014) Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl Med 3:18–31. https://doi.org/10.5966/sctm.2013-0110
Burridge PW, Matsa E, Shukla P et al (2014) Chemically defined generation of human cardiomyocytes. Nat Methods 11:855–860. https://doi.org/10.1038/nmeth.2999
Huang C, Ding T, Zhang Y et al (2023) The longevity protein p66Shc is required for neonatal heart regeneration. J Mol Cell Cardiol 177:21–27. https://doi.org/10.1016/j.yjmcc.2023.02.004
Chu Q, Jiang H, Zhang L et al (2020) CACCT: an automated tool of detecting complicated cardiac malformations in mouse models. Adv Sci (Weinh) 7:1903592. https://doi.org/10.1002/advs.201903592
Bodis K, Jelenik T, Lundbom J et al (2020) Expansion and impaired mitochondrial efficiency of deep subcutaneous adipose tissue in recent-onset type 2 diabetes. J Clin Endocrinol Metab 105:e1331-1343. https://doi.org/10.1210/clinem/dgz267
Phielix E, Jelenik T, Nowotny P, Szendroedi J, Roden M (2014) Reduction of non-esterified fatty acids improves insulin sensitivity and lowers oxidative stress, but fails to restore oxidative capacity in type 2 diabetes: a randomised clinical trial. Diabetologia 57:572–581. https://doi.org/10.1007/s00125-013-3127-2
Merino D, Villar AV, Garcia R et al (2016) BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc Res 110:331–345. https://doi.org/10.1093/cvr/cvw076
Au-Yeung CL, Yeung TL, Achreja A et al (2020) ITLN1 modulates invasive potential and metabolic reprogramming of ovarian cancer cells in omental microenvironment. Nat Commun 11:3546. https://doi.org/10.1038/s41467-020-17383-2
Huang Z, Sun D, Hu JX et al (2016) Neogenin promotes BMP2 activation of YAP and Smad1 and enhances astrocytic differentiation in developing mouse neocortex. J Neurosci 36:5833–5849. https://doi.org/10.1523/JNEUROSCI.4487-15.2016
Menzel J, di Giuseppe R, Biemann R et al (2017) Association between chemerin, omentin-1 and risk of heart failure in the population-based EPIC-Potsdam study. Sci Rep 7:14171. https://doi.org/10.1038/s41598-017-14518-2
Sam F, Duhaney TA, Sato K et al (2010) Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology 151:322–331. https://doi.org/10.1210/en.2009-0806
Kubin T, Poling J, Kostin S et al (2011) Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9:420–432. https://doi.org/10.1016/j.stem.2011.08.013
Talman V, Teppo J, Poho P et al (2018) Molecular atlas of postnatal mouse heart development. J Am Heart Assoc 7:e010378. https://doi.org/10.1161/JAHA.118.010378
Uosaki H, Cahan P, Lee DI et al (2015) Transcriptional landscape of cardiomyocyte maturation. Cell Rep 13:1705–1716. https://doi.org/10.1016/j.celrep.2015.10.032
Carrion M, Frommer KW, Perez-Garcia S, Muller-Ladner U, Gomariz RP, Neumann E (2019) The adipokine network in rheumatic joint diseases. Int J Mol Sci 20:4091. https://doi.org/10.3390/ijms20174091
Liu H, Wu J, Wang H, Sheng L, Tang N, Li Y, Hao T (2015) Association of serum omentin-1 concentrations with the presence and severity of preeclampsia. Ann Clin Biochem 52:245–250. https://doi.org/10.1177/0004563214541247
Tahmasebpour N, Hosseinpour Feizi MA, Ziamajidi N, Pouladi N, Montazeri V, Farhadian M, Abbasalipourkabir R (2020) Association of Omentin-1 with oxidative stress and clinical significances in patients with breast cancer. Adv Pharm Bull 10:106–113. https://doi.org/10.15171/apb.2020.013
Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, Randeva HS (2008) Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 57:801–808. https://doi.org/10.2337/db07-0990
Wang Q, Feng X, Zhou C, Li P, Kang J (2013) Decreased levels of serum omentin-1 in patients with obstructive sleep apnoea syndrome. Ann Clin Biochem 50:230–235. https://doi.org/10.1177/0004563212473275
Zhou JY, Chan L, Zhou SW (2014) Omentin: linking metabolic syndrome and cardiovascular disease. Curr Vasc Pharmacol 12:136–143. https://doi.org/10.2174/1570161112999140217095038
Çimen AR, Cerit ET, Iyidir OT et al (2017) Serum omentin-1 levels and endothelial dysfunction in obesity. Acta Endocrinol (Buchar) 13:138–143. https://doi.org/10.4183/aeb.2017.138
Zengi S, Zengi O, Kirankaya A, Kucuk SH, Kutanis EE, Yigit O (2019) Serum omentin-1 levels in obese children. J Pediatr Endocrinol Metab 32:247–251. https://doi.org/10.1515/jpem-2018-0231
Zhang Q, Zhu L, Zheng M et al (2014) Changes of serum omentin-1 levels in normal subjects, type 2 diabetes and type 2 diabetes with overweight and obesity in Chinese adults. Ann Endocrinol (Paris) 75:171–175. https://doi.org/10.1016/j.ando.2014.04.013
Rothermel J, Lass N, Barth A, Reinehr T (2020) Link between omentin-1, obesity and insulin resistance in children: Findings from a longitudinal intervention study. Pediatr Obes 15:e12605. https://doi.org/10.1111/ijpo.12605
Yang HY, Ma Y, Lu XH et al (2015) The correlation of plasma omentin-1 with insulin resistance in non-obese polycystic ovary syndrome. Ann Endocrinol (Paris) 76:620–627. https://doi.org/10.1016/j.ando.2015.08.002
Watanabe K, Watanabe R, Konii H et al (2016) Counteractive effects of omentin-1 against atherogenesis†. Cardiovasc Res 110:118–128. https://doi.org/10.1093/cvr/cvw016
Menzel J, Di Giuseppe R, Biemann R et al (2016) Association between omentin-1, adiponectin and bone health under consideration of osteoprotegerin as possible mediator. J Endocrinol Invest 39:1347–1355. https://doi.org/10.1007/s40618-016-0544-3
Yin J, Hou P, Wu Z, Nie Y (2015) Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med Sci Monit 21:118–122. https://doi.org/10.1265/msm.892081
Jin Z, Xia F, Dong J et al (2021) Omentin-1 attenuates glucocorticoid-induced cardiac injury by phosphorylating GSK3β. J Mol Endocrinol 66:273–283. https://doi.org/10.1530/jme-20-0236
Zhou JP, Tong XY, Zhu LP et al (2017) Plasma Omentin-1 level as a predictor of good coronary collateral circulation. J Atheroscler Thromb 24:940–948. https://doi.org/10.5551/jat.37440
Ji H, Wan L, Zhang Q, Chen M, Zhao X (2019) The effect of omentin-1 on the proliferation and apoptosis of colon cancer stem cells and the potential mechanism. J buon 24:91–98
Li D, Mei H, Pu J et al (2015) Intelectin 1 suppresses the growth, invasion and metastasis of neuroblastoma cells through up-regulation of N-myc downstream regulated gene 2. Mol Cancer 14:47. https://doi.org/10.1186/s12943-015-0320-6
Li D, Zhao X, Xiao Y et al (2015) Intelectin 1 suppresses tumor progression and is associated with improved survival in gastric cancer. Oncotarget 6:16168–16182. https://doi.org/10.18632/oncotarget.3753
Zhang YY, Zhou LM (2013) Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol 698:137–144. https://doi.org/10.1016/j.ejphar.2012.11.016
Kazama K, Okada M, Yamawaki H (2014) A novel adipocytokine, omentin, inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell migration through antioxidative mechanism. Am J Physiol Heart Circ Physiol 306:H1714-1719. https://doi.org/10.1152/ajpheart.00048.2014
Zhao LR, Du YJ, Chen L et al (2015) Omentin-1 promotes the growth of neural stem cells via activation of Akt signaling. Mol Med Rep 11:1859–1864. https://doi.org/10.3892/mmr.2014.2937
Yin L, Huang D, Liu X, Wang Y, Liu J, Liu F, Yu B (2017) Omentin-1 effects on mesenchymal stem cells: proliferation, apoptosis, and angiogenesis in vitro. Stem Cell Res Ther 8:224. https://doi.org/10.1186/s13287-017-0676-1
Cabral VLF, Wang F, Peng X et al (2022) Omentin-1 promoted proliferation and ameliorated inflammation, apoptosis, and degeneration in human nucleus pulposus cells. Arch Gerontol Geriatr 102:104748. https://doi.org/10.1016/j.archger.2022.104748
Wu SS, Liang QH, Liu Y, Cui RR, Yuan LQ, Liao EY (2013) Omentin-1 stimulates human osteoblast proliferation through PI3K/Akt signal pathway. Int J Endocrinol 2013:368970. https://doi.org/10.1155/2013/368970
Yang RZ, Lee MJ, Hu H et al (2006) Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 290:E1253-1261. https://doi.org/10.1152/ajpendo.00572.2004
de Souza Batista CM, Yang RZ, Lee MJ et al (2007) Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56:1655–1661. https://doi.org/10.2337/db06-1506
Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA (2013) Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J Clin Endocrinol Metab 98:E514-517. https://doi.org/10.1210/jc.2012-3673
Pan HY, Guo L, Li Q (2010) Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract 88:29–33. https://doi.org/10.1016/j.diabres.2010.01.013
Auguet T, Quintero Y, Riesco D et al (2011) New adipokines vaspin and omentin. circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med Genet 12:60. https://doi.org/10.1186/1471-2350-12-60
Li Z, Zhang Y, Tian F, Wang Z, Song H, Chen H, Wu B (2020) Omentin-1 promotes mitochondrial biogenesis via PGC1α-AMPK pathway in chondrocytes. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2020.1819337
Groppe J, Greenwald J, Wiater E et al (2002) Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420:636–642. https://doi.org/10.1038/nature01245
Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L (2003) Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 23:7230–7242. https://doi.org/10.1128/MCB.23.20.7230-7242.2003
Cao Y, Wang Y, Zhou Z et al (2022) Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science 377:1399–1406. https://doi.org/10.1126/science.abn0910
Dale JK, Vesque C, Lints TJ, Sampath TK, Furley A, Dodd J, Placzek M (1997) Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 90:257–269. https://doi.org/10.1016/s0092-8674(00)80334-7
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9:2808–2820. https://doi.org/10.1101/gad.9.22.2808
Tseng YH, Kokkotou E, Schulz TJ et al (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004. https://doi.org/10.1038/nature07221
Zeisberg EM, Tarnavski O, Zeisberg M et al (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13:952–961. https://doi.org/10.1038/nm1613
Tan CY, Wong JX, Chan PS et al (2019) Yin Yang 1 suppresses dilated cardiomyopathy and cardiac fibrosis through regulation of Bmp7 and Ctgf. Circ Res 125:834–846. https://doi.org/10.1161/CIRCRESAHA.119.314794
Murphy SA, Miyamoto M, Kervadec A et al (2021) PGC1/PPAR drive cardiomyocyte maturation at single cell level via YAP1 and SF3B2. Nat Commun 12:1648. https://doi.org/10.1038/s41467-021-21957-z
Acknowledgements
We are grateful to Dr. Hui Xie (Movement System Injury and Repair Research Center, Xiangya Hospital, Changsha, China) for the generation and provision of omentin-1−/− mice. We acknowledge the experimental research center of the Chinese Academy of Medical Sciences for the use of the IonOptix Systems. We also acknowledge the service of the public laboratory platform at the State Key Laboratory of Cardiovascular Disease for their assistance in flow cytometry (Shuo Gao), tissue sectioning (Jian Meng and Zhenyu Xu), and CardioExcyte 96 function analysis experiments (Kejia Zhong). We are grateful Weijing Liu, Zheng Qiao, Chiyin Wang, Wenlong Zhang, Shijie Sun, Haorui Liu, Wenzheng Chen, Zehao Yao, Yunxiaoxiao Wu, Hao Wang, Yuan Zhang, Yuan Liu, and Shanshan Xu for the proof, and polishing of the manuscript.
Funding
This work was supported by the National Key Research and Development Project of China (grant number 2019YFA0801500), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CAMS-I2M, 2021-I2M-1–072; 2021-I2M-C&T-A-011), the National Natural Science Foundation of China (grant number 81873509, 81970243, 81822004, 81670267, 81873479, and 31801068), and the Innovation-driven Project of Central South University (No. 2020CX017).
Author information
Authors and Affiliations
Contributions
HY conceived the project, performed experiments, analyzed data, and wrote the manuscript. SS analyzed RNA-seq data and edited the manuscript. JL and JF performed primary CM isolation experiments. YL performed apical resection models. ZC performed echocardiography. QS and XQ performed animal experiments. XB and XL rewrote and revised the manuscript. HL and LL analyzed data. YB and GZ participated in project design. YN designed and planned the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The animal experiments were conducted in accordance with the Guide for the Use and Care of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC), Fuwai Hospital, Chinese Academy of Medical Sciences.
Consent for publication
All the authors have consented to publication in CMLS.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file11 (MP4 5137 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Song, S., Li, J. et al. Omentin-1 drives cardiomyocyte cell cycle arrest and metabolic maturation by interacting with BMP7. Cell. Mol. Life Sci. 80, 186 (2023). https://doi.org/10.1007/s00018-023-04829-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04829-1