Abstract
A series of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives (1–12) was tested for their, in vitro antimycobacterial activity against Mycobacterium tuberculosis, and compound 2 was found to be more active than isoniazid. The antiviral screening results indicated that none of the tested compounds was active against a broad variety of DNA and RNA viruses at subtoxic concentrations, except compounds 8 and 10 that proved to be active against DNA viruses at concentrations close to their cytostatic potential. The synthesized compounds were also screened for their antimicrobial potential against S. aureus, B. subtilis, E. coli, C. albicans and A. niger, and the results indicated that compounds having Br, OCH3 and Cl groups were highly active. The multi-target QSAR models indicated the importance of lipophilic (log P) and topological parameters (3χv) in describing the antimicrobial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past few decades, the human population has been affected with life-threatening infectious diseases caused by multidrug-resistant Gram-positive and Gram-negative pathogenic bacteria which had increased at an alarming rate around the world. For this reason, new classes of antibacterial agents with novel mechanisms are crucially needed to combat with the multidrug-resistant infections (Bayrak et al., 2009a).
Tuberculosis (TB) is one of the leading infectious causes of death in the world and has re-emanated as a growing global health problem. This is not only because of the lack of proper therapeutic agents for its treatment but also because of development of drug-resistant strains (Kamal et al., 2006). The resurgence of TB over the last 15 years, even in industrialized countries where it had almost been eradicated, has been favored by the pathogenic synergy with human immunodeficiency virus (HIV) infection. In fact, TB and other atypical mycobacterias are now diseases frequently associated with AIDS; HIV infection significantly increases the risk that new or latent TB infections will progress to active diseases (Shahar Yar et al., 2007). However, powerful new antitubercular drugs with new mechanisms of action have not been developed over the last 40 years. In the developing countries, the annual infection rate is 20–50 times greater than in the developed countries, and this prevailing high level shows little or no downward trend. It is expected that development of new effective anti-TB drugs will bring various outcomes, viz., shortening the total duration of therapy, reducing the total expenditure and treatment of multidrug-resistant tuberculosis (MDR-TB) by single dosage regimen (Maccari et al., 2002).
Over the last decade, great strides have been made in the treatment of HIV infection through use of drug combinations known as highly active anti-retroviral therapy (HAART). HAART regimens usually include three or more drugs with inhibitory activity against either HIV reverse transcriptase (RT) or HIV protease. Currently, most HAART regimens include a backbone of two nucleos(t)ide reverse-transcriptase inhibitors (N(t)RTIs) with a third agent added from the non-nucleoside reverse-transcriptase inhibitor (NNRTI) or protease inhibitor classes (PI) (Boojamra et al., 2008). Although combination therapies have proven to decrease HIV-related mortality, there is still a greater need for development of novel antiretroviral agents due to the emergence of multi-drug resistance, which is a major challenge to successful therapy for individuals infected with HIV (Kucukguzel et al., 2008).
A large number of constitutional, topological, geometric, electrostatic, and quantum indices were introduced with the aim to express the chemical structure in a numerical form. Such structural descriptors can be utilized to model physical, chemical, or biological properties with quantitative structure–property relationships (QSPRs) and quantitative structure–activity relationships (QSARs) (Ivanciuc et al., 2002). Spatial descriptors describe the molecule’s ‘‘solvent-accessible’’ surface areas and their charges. Electronic descriptors describe the electron orientation and charge. Topological descriptors are based on graphic/structure concepts and geometric features such as shape, size, and branching. Thermodynamic descriptors describe energy of molecules and their conversions. Quantum mechanical descriptors are calculated using semi-empirical methods that are likely to be more accurate (Sahu et al., 2007).
Isoniazid is a prodrug that targets mycolic acid biosynthesis and is activated inside the mycobacterial cell. Isoniazid is activated either due to the isonicotinic acyl anion (Shoeb et al., 1985) or radical (Johnson and Schultz, 1994) by KatG, a catalase–peroxidase enzyme (Zhang et al., 1992). When activated by the catalase–peroxidase katG, INH attaches itself to NADH to form a covalent adduct INH-NAD. This is the actual drug that binds the inhA enzyme and inhibits its function (Khasnobis et al., 2002). The antimicrobial activity of isoniazid derivatives have been investigated very recently (Bayrak et al., 2009a; Bayrak et al., 2009b; Rodriguez-Arguelles et al., 2007). Overwhelmed by all these facts and in continuation of our research endowed toward the synthesis, antimicrobial, antimycobacterial, antiviral and QSAR studies (Kumar et al., 2010a, b, 2007; Judge et al., 2010), we hereby report the synthesis, antitubercular, anti-HIV, antimicrobial, and QSAR studies of Isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides.
Experimental
Melting points were determined in open capillary tubes on a Sonar melting point apparatus and are uncorrected. Reaction progress was monitored by thin layer chromatography on silica gel sheets (Merck silica gel-G), and the purity of the compounds was ascertained by single spot on TLC sheet. 1H nuclear magnetic resonance (1H NMR) spectra were recorded in Bruker Avance II 400 NMR spectrometer using appropriate deuterated solvents and are expressed in parts per million (δ, ppm) downfield from tetramethylsilane (internal standard). Infrared (IR) spectra were recorded on a Shimadzu FTIR spectrometer.
General procedure for synthesis of ester of isonicotinic acid
The mixture of isonicotinic acid (0.1 mol) and ethanol (in excess) was refluxed with sulfuric acid (1–2 ml) till the completion of reaction monitored by TLC on silica gel G plates. Then, the reaction mixture was added to 200 ml of ice cold water, and excess of the acid was neutralized by a solution of sodium bicarbonate. The crude ester was extracted with ether. The ether layer was separated, and ester was obtained on evaporation of ether layer.
General procedure for the synthesis of isonicotinic acid hydrazide
The ethanolic solution of ester (0.01 mol) and hydrazine-hydrate (0.015 mol) was refluxed for 6–7 h. The reaction mixture was then cooled, and the precipitates were washed with water, dried, and recrystallized from ethanol.
General procedure for the synthesis of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides (1–12)
The solution of isonicotinic acid hydrazide (0.01 mol) and appropriate substituted acetophenone/cycloheptanone (0.01 mol), in ethanol, was refluxed for 4–5 h. The precipitates obtained were filtered off, washed, and recrystallized from ethanol.
Isonicotinic acid [1-(4-bromo-phenyl)-ethylidene]-hydrazide (2)
Mp (°C) 237–240; Yield 72.6%; 1H NMR (400 MHz, CDCl3) δ ppm: 2.19 (s, 3H, CH3), 7.52–7.65 (m, 4H, CH of phenyl ring), 7.78–7.91 (d, 2H, CH of C3 and C5 of pyridine ring; J = 12), 8.78–8.81 (d, 2H, CH of C2 and C6 of pyridine ring; J = 12), and 11.71 (s, 1H, NH); 13CNMR (δ ppm): 11.4, 121.6, 121.9, 125.4, 129.3, 130.5, 130.8, 131.4,141.6, 149.0, 154.2, and 168.4; IR (KBr pellets) ν cm−1: 3186.54 (NH str., amide), 3074.66 (CH str., aromatic), 1670.43 (C=O str., amide), 1600.99 (C=C str., skeletal phenyl nucleus), 825.57 (CH out-of-plane bending, 4-substituted pyridine), and 597.96 (C–Br str., aromatic); CHN analysis calc.(found): C, 52.85 (52.19); H, 3.80 (3.65); N, 13.21 (13.32); O, 5.03 (4.91); and Br, 25.11 (24.95).
Isonicotinic acid [1-(2,4-dichloro-phenyl)-ethylidene]-hydrazide (3)
Mp (°C) 175–178; Yield 55.4%; 1H NMR (400 MHz, DMSO) δ ppm: 2.35 (s, 3H, CH3), 7.25–7.46 (m, 3H, CH of C3, C5 and C6 of dichloro phenyl ring), 7.74–7.75 (d, 2H, CH of C3 and C5 of pyridine ring; J = 4), 8.70–8.71 (d, 2H, CH of C2 and C6 of pyridine ring; J = 4), and 11.00 (s, 1H, NH); 13CNMR (δ ppm): 11.2, 121.3, 121.8, 126.5,128.9, 129.3, 134.2, 136.7, 141.2, 149.5, 154.7, and 169.1; IR (KBr pellets) ν cm−1: 3187.51 (NH str., amide), 3031.26 (CH str., aromatic), 1661.75 (C=O str., amide), 1559.51 (C=C str., skeletal phenyl nucleus), 816.89 (CH out of plane bending, 4-substituted pyridine), and 666.43 (C–Cl str., aromatic); CHN analysis calc.(found): C, 54.57 (54.38); H, 3.60 (3.81); N, 13.64 (13.49); O, 5.19 (5.03); Cl, and 23.01 (22.87).
Isonicotinic acid [1-(4-nitro-phenyl)-ethylidene]-hydrazide (4)
Mp (°C) Above 242; Yield 87.3%; 1H NMR (400 MHz, DMSO) δ ppm: 2.14 (s, 3H, CH3), 7.62–7.81 (m, 4H, CH of phenyl ring), 8.24–8.26 (d, 2H, CH of C3 and C5 of pyridine ring; J = 8), 8.68–8.77 (d, 2H, CH of C2 and C6 of pyridine ring), and 11.15 (s, 1H, NH); 13CNMR (δ ppm): 11.4, 121.4, 121.8, 122.7, 123.2, 128.7, 129.3, 136.9, 141.8, 149.1, 150.2, 154.2, and 168.5; IR (KBr pellets) ν cm−1: 3191.36 (NH str., amide), 3091.06 (CH str., aromatic), 2937.71 (CH str., aliphatic), 1671.39 (C=O str., amide), 1577.84 (C=C str., skeletal phenyl nucleus), 1508.40 (NO2 str. asymmetric, aromatic nitro group), 1350.23 (NO2 str. symmetric, aromatic nitro group), and 843.89 (CH out of plane bending, 4-substituted pyridine); CHN analysis calc.(found): C, 59.15 (58.91); H, 4.25 (4.11); N, 19.71 (19.87); O, and 16.88 (16.63).
Isonicotinic acid [1-(2,4-dimethoxy-phenyl)-ethylidene]-hydrazide (6)
Mp (°C) 120–123; Yield 59.1%; 1H NMR (400 MHz, DMSO) δ ppm: 2.07 (s, 3H, CH3), 3.79 (s, 6H, OCH3), 6.58 (s, 1H, CH of C3 of phenyl ring), 7.31–7.34 (d, 1H, CH of C5 of phenyl ring; J = 12), 7.40–7.42 (d, 1H, CH of C6 of phenyl ring; J = 8), 7.69–7.71 (d, 2H, CH of C3 and C5 of pyridine ring; J = 8), 8.60–8.63 (d, 2H, CH of C2 and C6 of pyridine ring; J = 12), and 10.72 (s, 1H, NH); 13CNMR (δ ppm): 11.8, 55.6, 55.9, 100.3, 106.5, 109.8, 122.2, 122.7, 130.8, 142.0, 149.2, 149.6, 153.5, 163.1, 164.8, and 169.2; IR (KBr pellets) ν cm−1: 3105.53 (NH str., amide), 1683.63 (C=O str., amide), 1307.79 (C–O–C str., aralkyl ether), and 792.78 (CH out of plane bending, 4-substituted pyridine); CHN analysis calc.(found): C, 64.20 (64.32); H, 5.72 (5.51); N, 14.04 (13.89); and O, 16.04 (16.23).
Isonicotinic acid [1-(4-hydroxy-phenyl)-ethylidene]-hydrazide (9)
Mp (°C) Above 242; Yield 76.0%; 1H NMR (400 MHz, DMSO) δ ppm: 2.15 (s, 3H, CH3), 6.75–6.77 (s, 1H, OH), 6.83–6.87 (d, 2H, CH of C3 and C5 of phenyl ring; J = 16), 7.74–7.76 (d, 2H, CH of C2 and C6 of phenyl ring; J = 8), 7.80–7.84 (d, 2H, CH of C3 and C5 of pyridine ring; J = 16), 8.75–8.76 (d, 2H, CH of C2 and C6 of pyridine ring; J = 4), and 10.69 (s, 1H, NH); 13CNMR (δ ppm): 11.5, 114.2, 114.5,121.4, 121.9, 123.1,129.4, 129.7, 141.5,149.3, 154.1, 159.2, and 168.3; IR (KBr pellets) ν cm−1: 3666.84 (OH str., Phenol), 3279.13 (NH str., amide), 3068.88 (CH str., aromatic), 2795.94 (CH3 str. asymmetric, ArCH3), 2685.99 (CH3 str. symmetric, ArCH3), 1647.28 (C=O str., amide), and 1532.51 (C=C str., skeletal phenyl nucleus), 826.53 (CH out of plane bending, 4-substituted pyridine); CHN analysis calc.(found): C, 65.87 (65.63); H, 5.13 (5.01); N, 16.46 (16.23); and O, 12.54 (12.38).
Evaluation of antimycobacterial activity
All compounds were screened for their in vitro antimycobacterial activity against MTB, in Middlebrook 7H11agar medium supplemented with OADC by agar dilution method similar to that recommended by the National Committee for Clinical Laboratory Standards for the determination of MIC in triplicate (National Committee for Clinical Laboratory Standards, 1995). The minimum inhibitory concentration (MIC) is defined as the minimum concentration of compound required to give complete inhibition of bacterial growth.
Evaluation of antimicrobial activity (determination of minimum inhibitory concentration)
The antimicrobial activity was performed against Gram-positive bacteria: S. aureus and B. subtilis; Gram-negative bacterium: E. coli; and fungal strains: C. albicans and A. niger by tube dilution method (Cappucino and Sherman, 1999). Dilutions of test and standard compounds [norfloxacin (antibacterial) and fluconazole (antifungal)] were prepared in double strength nutrient broth—I.P. (bacteria), and Sabouraud dextrose broth I.P. (fungi) (Pharmacopoeia of India, 2007). The samples were incubated at 37°C for 24 h (bacteria), at 25°C for 7 days (A. niger) and at 37°C for 48 h (C. albicans), respectively, and the results were recorded in terms of MIC (the lowest concentration of test substance which inhibited the growth of microorganisms).
QSAR studies
The structures of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives were first pre-optimized with the Molecular Mechanics Force Field (MM+) procedure included in Hyperchem 6.03 (Hyperchem 6.0, 1993), and the resulting geometries are further refined by means of the semiempirical method PM3 (Parametric Method-3). We chose a gradient norm limit of 0.01 kcal/Å for the geometry optimization. The lowest energy structure was used for each molecule to calculate physicochemical properties using TSAR 3.3 software for Windows (TSAR 3D Version 3.3, 2000). Further, the regression analysis was performed using the SPSS software package (SPSS for Windows, 1999).
Calculation of statistical parameters
The developed QSAR models were validated by the calculation of following statistical parameters : probable error of the coefficient of correlation (PE), least square error (LSE), Friedman’s lack of fit measure (LOF), standard error of prediction (SEP), quality value (Q), and SSY (sum of squares of response values) (Mandloi et al., 2005; Pinheiro et al., 2004).
These parameters were calculated from the following equations:
where, r is the correlation coefficient, and n is the number of compounds used.
where, Y obs and Y calc are the observed and calculated values.
where, LSE is the least square error; C is the number of descriptors +1; p is the number of independent parameters; n is the number of compounds used; and d is the smoothing parameter which controls the bias in the scoring factor between equations with different number of terms, and was kept 1.0.
The Quality value, Q is given by
where, Q is the Quality value, r is the correlation coefficient, and Se is standard error.
The predictive ability of QSAR models was also quantified in terms of q 2, which is defined as
The low values of PE, LSE, LOF, and SEP and the high values of Q and q 2 are the essential criteria for qualifying the model as the best one.
Evaluation of anti-HIV activity
The anti-HIV activity and cytotoxicity were evaluated against HIV-1 strain IIIB and HIV-2 strain ROD in MT-4 cell cultures using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method (Pauwels et al., 1988). In brief, virus stocks were titrated in MT-4 cells and expressed as the 50% cell culture infective dose (CCID50). MT-4 cells were suspended in culture medium at 1 × 105 cells/ml and infected with HIV at a multiplicity of infection of 0.02. Immediately after viral infection, 100 μl of the cell suspension was placed in each well of a flat-bottomed microtiter tray containing various concentrations of the test compounds. After a 4-day incubation period at 37°C, the number of viable cells was determined using the MTT method. Compounds were tested in parallel for cytotoxic effects in uninfected MT-4 cells.
Antiviral assays
The antiviral assays [except anti-HIV assays] were based on inhibition of virus-induced cytopathicity in HEL [herpes simplex virus type 1 (HSV-1), HSV-2 (G), vaccinia virus, and vesicular stomatitis virus], Vero (parainfluenza-3, reovirus-1, Sindbis, Coxsackie B4, and Punta Toro virus), HeLa (vesicular stomatitis virus, Coxsackie virus B4, and respiratory syncytial virus) cell cultures. Confluent cell cultures in microtiter 96-well plates were inoculated with 100-cell culture inhibitory dose-50 (CCID50) of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) in the presence of varying concentrations (100, 20, 4,… μg/ml) of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds.
Results and discussion
Chemistry
The synthesis of target compounds was carried out as depicted in Scheme 1. The isonicotinic acid was refluxed with ethanol in the presence of sulphuric acid to get the ethyl ester of isonicotinic acid. The ester thus obtained was refluxed with hydrazine hydrate to obtain the isonicotinic acid hydrazide. The isonicotinoyl hydrazide was refluxed with various substituted acetophenones/cycloheptanone to yield the target isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides (1–12). The low yield of some synthetic compounds may be attributed to any one or more of the following reasons (Furniss et al., 1998): (a) the reaction may be reversible and position of equilibrium is unfavorable to the product; (b) the incursion of side reactions leading to the formation of by-products; (c) the premature work-up of the reaction before its completion; (d) the volatilization of products during reaction or work-up; (e) the loss of product due to incomplete extraction, inefficient crystallization, or other work-up procedures; (f) the presence of contaminants in the reactants or reagents leading to a less efficient reaction. In addition to the above facts, the compound 11 has been derived from cycloheptanone which is a cyclic ketone, due to which it may be less reactive toward the reaction with hydrazide which may have led to the lower yield. In compound 12, where Br group is present at ortho position, the electronegative Br group creates difficulty in the release of ketonic oxygen as an anion and, hence, lowers the reaction rate that may have led to the lesser yield of this compound. The physicochemical characteristics of synthesized compounds are presented in Table 1.
The structures of synthesized compounds were confirmed on the basis of their consistent IR and NMR spectral data. The presence of singlet signal above δ 10.69 ppm in compounds 2, 3, 4, 6, and 9 depicted the presence of NH proton of hydrazide linkage in the synthesized derivatives. The presence of singlet signal around δ 2.07–2.35 ppm revealed the presence of CH3 group of acetophenones in the synthesized compounds. In all the synthesized compounds, two doublet signals were observed at two different δ values, with the one at higher δ value corresponding to C2 and C6 CH protons as they are in close proximity of the electronegative nitrogen atom in the ring and the doublet signal at lower δ represents the remaining two protons of the isonicotinyl nucleus. The singlet signal having six protons due to OCH3 at δ 3.79 ppm in compound 6 confirmed the presence of a dimethoxy group in the synthesized compound. The appearance of signal for four aromatic protons in compounds 2, 4, and 9 and signals for three aromatic protons in compounds 3 and 6 confirmed the presence of a monosubstituted and disubstituted phenyl nucleus in the synthesized compound, respectively. The singlet signal at δ 4.46 ppm due to OH protons in compound 9 confirmed the presence of hydroxyl group in its structure.
The presence of C=O functional group was demonstrated by the appearance of stretching band around 1670 cm−1, in compounds 2, 3, 4, 6, and 9, which is the characteristic of the amide linkage. In compounds 2, 3, 4, 6, and 9, the characteristic NH stretching band of a secondary amide was observed around 3200–3100 cm−1. The presence of peaks slightly above and below 3000 cm−1 indicates the presence of an aromatic and aliphatic portions in synthesized compounds, respectively. The skeletal C=C– stretching bands were observed around 1500 cm−1 in the spectra of the synthesized compounds which represents the presence of aromatic groups. In compound 6, the C–O–C stretching band was observed at 1307.79 cm−1, which revealed the presence of methoxy group in its structure.
The CH out-of-plane bending observed around vibrational frequency of 860–814 cm−1 indicated the presence of a 4-substituted pyridine ring in the structure of synthesized compounds. The characteristic C–Br str. and C–Cl str. bands were observed at 597.96 and 666.43 cm−1 in compounds 2 and 3, respectively. The presence of OH group in compound 9 was confirmed by a stretching band at 3666.84 cm−1. The asymmetric NO2 str. at 1508.40 cm−1 and symmetric NO2 str. at 1350.23 cm−1 confirmed the presence of an aromatic nitro group in compound 4.
Antimycobacterial activity
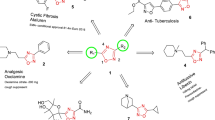
The in vitro antitubercular activity against Mycobacterium tuberculosis (MTB) was carried out in Middlebrook 7H11agar medium supplemented with OADC by agar dilution method, and the results are presented in Table 1. Compound 1, having chloro substitution at para position, had shown excellent antimycobacterial activity having MIC of 3 × 10−3 μM. Based on these results, we planned to substitute some other electronegative atom in the molecules, and synthesized compound 2, having p-bromo substitution on the phenyl nucleus; it showed improved antimycobacterial activity with MIC value 2 × 10−3 μM, but when bromo substituent was added in ortho position (compound 12), a fall in activity was observed with MIC of 5 × 10−3 μM, showing that para substitution favors the antimycobacterial activity. As there was an improvement of antimycobacterial activity with electronegative substituents, this stimulated us to substitute the phenyl nucleus with two electronegative atoms in compound 3, having chloro substituents at ortho and para positions, but improvement in activity was not observed, and it showed antimycobacterial activity at 2 × 10−3 μM. Then, we planned to introduce more electron-withdrawing nitro group as substituent in the synthesized molecules and came out with compounds 4 and 5 having nitro substitution at para and meta positions, respectively. Compound 4 was highly active with an MIC value <3 × 10−3 μM, and compound 5 had an MIC value of 5 × 10−3 μM, which indicates that an electron-withdrawing group at meta position decreased the activity. The next change in the substituent was the addition of an electron-donating methoxy group in compounds 6 and 7, but, unfortunately, this change led to a fall in antimycobacterial activity. Compound 6 having 2,4-dimethoxy substituent had MIC of 10 × 10−3 μM and compound 7 had an MIC of 21 × 10−3 μM. Then, we added hydroxyl substituent in the phenyl nucleus and synthesized compounds 8, 9, and 10. Again, a steep fall in activity was observed upon addition of a hydroxy substituent in the phenyl ring, in compounds 8, 9, and 10, at ortho, para, and ortho-para positions, respectively. In compound 11, the phenyl nucleus was replaced with cycloheptyl ring, and a highly active molecule with an MIC of 3 × 10−3 μM was obtained. It is seen from the results of antimycobacterial activity that compounds 1–5 and 11 were found to be highly active with MIC values <5 × 10−3 μM, which is well below the MIC values of standard drugs ciprofloxacin and ethambutol. Compound 2 was found to be a more active antimycobacterial agent than isoniazid, and compound 3 was also found to be equally active.
From the results of antimycobacterial activity, the following conclusions regarding structure–activity relationship (SAR) can be drawn:
-
1.
The presence of electron-withdrawing groups on the phenyl ring increases the antitubercular activity as evidenced by the high antimycobacterial activity of compounds 1–5. The role of electron-withdrawing group in improving antimicrobial activities is supported by the studies of Sharma et al. (2004)
-
2.
The presence of OH group at ortho position (Compound 8) increases the antimycobacterial activity in comparison to the presence of OH group at para position (Compound 9). This is in concordance with Tripathi et al. (2006) who stated that OH group at ortho position leads to a measurable change in activity of the compounds.
-
3.
The substitution of phenyl ring with electron-donating OCH3 (Compound 5) decreases the antimycobacterial activity. These results are similar to the reports of Sriram et al. (2007) who observed that the addition of methoxy group decreases the antimycobacterial activity of N-hydroxythiosemicarbazones.
-
4.
The replacement of benzylidene ring with cycloheptylidene ring (compound 11) improves the antimycobacterial activity of the molecule.
-
5.
The presence of halo groups at para position improved the antitubercular activity of the synthesized compounds. This is similar to the results observed by Mamolo et al. (2004) who stated that the presence of bromo, chloro, and phenyl substituents, at the para position of the benzene ring improved the antitubercular activity.
-
6.
The presence of electron-withdrawing bromo group in compound 2 enhances the growth-inhibiting potency of the molecule, which is similar to the results observed by Masunari and Tavares (2007) who stated that the presence of bromo and chloro substituents, at the para position of the benzene ring increases the activity.
-
7.
Compound 5 with nitro substitution at para position was more active than nitro substitution at ortho position (compound 6).
Cytostatic, cytotoxic and antiviral activity
In general, none of the compounds was inhibitory to a variety of DNA and RNA viruses tested at subtoxic concentrations (Tables 2, 3, 4, 5), except for compounds 8 and 10 that showed activities against HSV-1, HSV-2, and VV. Although the compounds were not measurably toxic against the HEL cell cultures (in which the anti-DNA virus activity was performed), they showed pronounced toxicity in several other cell types. Also, they were very cytostatic against HEL cell proliferation. Therefore, it is currently unclear whether the anti-DNA virus activity noticed is due to a specific antiviral activity or to a (more likely) indirect antiviral effect due to a cellular cytostatic potential of the compounds.
Antibacterial and antifungal activities
The in vitro antimicrobial activity of synthesized compounds were determined by tube dilution method using norfloxacin and fluconazole as reference standards for antibacterial and antifungal activities, respectively, and the results are presented in Table 6.
The results for antibacterial activity against B. subtilis indicated that compound 12 was the most active among all the synthesized derivatives as well as the standard drug norfloxacin. In particular, the compounds 6 and 12 were found to be the most effective antibacterial agents having pMICbs values 2.38 and 2.71 μM, respectively. In case of S. aureus, compounds 1, 6, and 12 have shown marked antibacterial potential at pMICsa values 2.34, 2.38, and 2.41 μM, respectively. For antibacterial activity against E. coli compounds 2, 7, and 12 were found to be effective with pMICec values of 2.41, 2.68, and 2.41 μM, respectively. The antibacterial potential of compound 7 against E. coli was higher than the activity of norfloxacin standard.
The antifungal activity against C. albicans demonstrated that compounds 3, 6, and 12 were the potential candidates having pMICca values 2.69, 2.68, and 2.71 μM, respectively. All these three compounds (3, 6, and 12) have better antifungal activities than the standard drug fluconazole, and compound 10 had shown the antifungal potential equivalent to the standard. In case of A. niger, compounds 2 and 12 were found to be active with pMICan value of 2.41 μM each, which is better than the parent drug isonicotinic acid hydrazide.
It is evident from the results presented in Table 6 that the synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives have shown marked antimicrobial potential against the tested strains, with some of the synthesized compounds exhibiting their antimicrobial activity higher than the standard drugs norfloxacin and fluconazole. The isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives were found to be more active against fungal strains C. albicans and A. niger, and Gram negative E. coli as compared with Gram-positive B. subtilis and S. aureus. These results of antimicrobial activity can be represented as follows:
C. albicans > A. niger > E. coli > B. subtilis > S. aureus
Structure activity relationship
From the results of antimicrobial activity, the following SARs can be drawn:
-
1.
The presence of substituent at ortho position increases the antibacterial and antifungal potentials of the compounds which are seen from the antimicrobial activity of compounds 6, 8, 10, and 12. This fact is supported by the observations of Guven et al. (2007).
-
2.
The compounds have shown marked antifungal potential as compared to antibacterial activity, which shows that different structural requirements are essential for antibacterial and antifungal activity. These results are similar to those of Sortino et al. (2007).
-
3.
The results of antimicrobial activity depicted that the presence of electron-donating group, OCH3, enhanced the antimicrobial activity of the synthesized derivatives (Compound 6 and 7). This is supported by the findings of Emami et al. (2008).
-
4.
The replacement of benzylidene nucleus with cycloheptylidene nucleus (Compound 11) leads to a decline in antimicrobial activity. This fact indicates that phenyl nucleus is necessary for antimicrobial activity.
-
5.
The synthesized derivatives were more active toward the Gram-negative bacterium E. coli than Gram-positive B. subtilis and S. aureus. This finding is in concordance with Sbardella et al. (2004).
-
6.
The results of antimicrobial activity showed that ortho- and para-disubstituted chloro derivative (compound 3) has higher antimicrobial potency in comparison to monochloro-substituted (compound 1) derivative except against S. aureus. The higher antimicrobial potential of compound 3 may be due to the presence of an extra electron-withdrawing chloro group in its structure.
-
7.
The presence of electron-withdrawing bromo group in compounds 2 and 12 enhances the growth inhibition potency of isonicotinic acid-1-(substituted phenyl)-ethylidene hydrazide derivatives. This is similar to the results observed by Masunari and Tavares (2007) who stated that the presence of bromo and chloro substituents, at the para position of the benzene ring showed marked antibacterial activity against S. aureus.
The SAR findings of the antimycobacterial and antimicrobial activity are summarized in Fig. 1.
QSAR studies
Development of one-target QSAR model
In order to identify the substituent effect on the antimicrobial activity, QSAR studies between the in vitro activity and descriptors coding for lipophilic, electronic, steric, and topological properties of the 12 synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives were undertaken, using the linear free-energy relationship model (LFER) described by Hansch and Fujita (1964). Biological activity data determined as MIC values was first converted into pMIC values (i.e., −log MIC) and used as dependent variable in QSAR study.
The different molecular descriptors (independent variables) like log of octanol–water partition coefficient (log P), molar refractivity (MR), Kier’s molecular connectivity (0χ, 0χv, 1χ, 1χv, 2χ, 2χv) and shape (κ1, κ2, κ3, κα1, κα2, κα3) topological indices, Randic topological index (R), Balaban topological index (J), Wiener topological index (W), Total energy (Te), energies of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), dipole moment (μ), nuclear repulsion energy (Nu.E), and electronic energy (Ele.E), calculated for isoniazid derivatives are presented in Table 7 (Hansch et al., 1973; Kier and Hall, 1976; Randic 1975; Balaban 1982; Wiener 1947; Randic 1993). Units of the energies and dipole were electron volts (eV), and atomic units (a.u.), respectively (Dai et al., 1999).
In the present study, a dataset of 13 compounds (12 isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives along with isoniazid itself) was subjected to linear/multiple linear free-energy regression analysis for model generation. During the regression analysis studies, it was observed that the response values of compound 12 were outside the limits of response values of other synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives. Thus, compound 12 was designated as an outlier, and it was not involved in the dataset for QSAR model generation. In multivariate statistics, it is common to define three types of outliers (Furusjo et al., 2006):
-
1.
X/Y relation outliers are substances for which the relationship between the descriptors (X variables) and the dependent variables (Y variables) is not the same as in the (rest of the) training data.
-
2.
X outliers. In brief, a substance is an X outlier if the molecular descriptors for this substance do not lie in the same range as the (rest of the) training data.
-
3.
Y outliers are only defined for training or test samples. They are substances for which the reference value of response is invalid.
In light of the above guidelines, compound 12 was considered as outlier because its response values [antimicrobial activity] were outside the range in comparison to the other compounds included in the present study.
Preliminary analysis was carried out in terms of correlation analysis. A correlation matrix constructed for antibacterial activity against A. niger is presented in Table 8. The correlations of different molecular descriptors with antibacterial and antifungal activities are presented in Table 9. In general, high colinearity (r > 0.8) was observed between different parameters. The high interrelationship was observed between 0χ and κ1 (r = 0.999), and the low interrelationship was observed between 3χv and μ (r = 0.220). The correlation matrix indicated the predominance of topological parameters in describing the antimicrobial activity of the synthesized compounds.
The antifungal activity of synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives against A. niger is governed by the third order molecular connectivity topological index (3χ) (Eq. 1).
QSAR model for antifungal activity against A. niger
where and henceforth, n is the number of data points, r is the correlation coefficient, q 2 is the cross-validated r 2 obtained by leave-one-out (LOO) method, s is the standard error of the estimate, and F is the Fischer statistics.
For antifungal activity against A. niger, the developed QSAR model (Eq. 1) describes the importance of third-order molecular connectivity topological index (3χ). Topological indices are numerical quantifiers of molecular topology and are sensitive to bonding pattern, symmetry, content of heteroatom as well as degree of complexity of atomic neighborhoods (Lather and Madan, 2005). The third-order molecular connectivity topological index (3χ) represents the molecules with highly branched structure. In this case, the positive correlation was observed between 3χ and antifungal activity against A. niger, and the results presented in the Table 9 are in concordance with the model expressed by Eq. 1. It can be seen from Table 7 that compounds 2, 3, 6, and 7 having high 3χ values (1.093, 1.299, 1.145, and 1.145) have got the highest antibacterial potential (2.41, 2.39, 2.38, and 2.38). The compound 11 having the least 3χ value (0.606) has got the minimum antifungal activity (1.66).
The QSAR model expressed by Eq. 1 was cross validated by q 2 values obtained with LOO method. The value of q 2 greater than 0.5 is the basic requirement for qualifying a QSAR model as the valid one (Golbraikh and Tropsha, 2002). In this case, the q 2 (Eq. 1) value is less than 0.5, which shows that the developed model is an invalid one. However, according to the recommendations of Kim et al. (2007), the regression models are acceptable if the value of standard deviation (SD, Table 6) is not much larger than 0.3. As the value of standard deviation in case of (Eq. 1) is less than 0.3 i.e., 0.21, so the developed model is a valid one. The comparisons of the observed and predicted antifungal activities are presented in Table 10. It is evident from the results that the observed and the predicted antibacterial activities lie close to each other as indicated by their low residual values (Table 10). The low residual activity values also support the validity of the QSAR model described by Eq. 1. The low values of PE, LSE, LOF, and SEP, and the high values of Q, (Table 11) revealed the statistical significance of the model described by Eq. 1.
Equations 2–5 were developed to predict the antibacterial and antifungal activities of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives against B. subtilis, E. coli and C. albicans.
QSAR model for antibacterial activity against B. Subtilis
QSAR model for antibacterial activity against E. coli
QSAR model for antifungal activity against C. albicans
QSAR model for antifungal activity against C. albicans obtained by MLR
The model expressed by Eq. 2 demonstrated that antibacterial activity against B. subtilis is governed by the Wiener topological index (W). The Wiener topological index (W) was introduced by Wiener (Wiener, 1947) to demonstrate the correlations between the physicochemical properties of organic compounds and the topological structure of their molecular graphs in terms of sum of distances between any two carbon atoms in the molecule.
As the coefficient of Wiener topological index (W) in Eq. 2 is positive, the antibacterial activity against B. subtilis will increase with increase in the Wiener topological index (W) values. This is clearly evident from Table 6 that compounds 4, 5, 6, and 7 having high W values of 1086.00, 1050.00, 1175.00, and 1155.00, respectively (Table 7), have got the highest antibacterial activity values of 2.06, 2.06, 2.38, and 2.08 respectively. Similarly, compound 11 having minimum W value 584.00 (Table 7) has got the minimum antibacterial activity against B. subtilis (Table 6).
The model described by Eq. 3 demonstrated the importance of third order molecular connectivity topological index (3χ) in describing the antibacterial activity against E. coli. The positive correlation of the molecular descriptor with antibacterial activity reveals that increase in the value of 3χ (Table 7) will lead to an increase in antibacterial activity against E. coli. The QSAR model for antifungal activity (Eq. 4) against C. albicans depicted that third-order molecular connectivity topological index (3χ) best describes the antifungal activity of synthesized derivatives. The model expressed by Eq. 4 has got low r value which stimulated us to go for the development of multiparametric models using multiple linear regression (MLR) analysis. Using the MLR analysis, we came out with Eq. 5, where combination of third-order molecular connectivity topological index (3χ) with dipole moment (μ) increased the r value from 0.568 to 0.712.
Similar to Eq. 1, Eqs. 2–5 have also got low q 2 values, hence in these cases also, the low standard deviation supports the validity of developed QSAR models. The low residual values presented in Table 10 also support the fact that models expressed by Eqs. 2–5 are valid ones. The low values of PE, LSE, LOF, and SEP and high value of Q, (Table 11) revealed the statistical significance of the model described by Eqs. 2, 3, and 5. Statistically significant models were not obtained for antibacterial activity against S. aureus.
In general, for QSAR studies, the biological activities of compounds should span 2–3 orders of magnitude. However, in the present study, the range of antibacterial and antifungal activities of the synthesized compounds is within one order of magnitude. It is important to note that the predictability of the QSAR models developed in the present study is high as evidenced by their low residual values. This is in accordance with results suggested by the Bajaj et al. (2005), who stated that the reliability of the QSAR model lies in its predictive ability, even though the activity data are in the narrow range. Further, the recent literature reveals that the QSAR have been applied to describe the relationship between narrow range of biological activity and physicochemical properties of the molecules (Narasimhan et al., 2007; Sharma et al., 2006; Hatya et al., 2006). When biological activity data lie in the narrow range, the presence of minimum standard deviation of the biological activity justifies its use in QSAR studies (Kumar et al., 2007; Narasimhan et al., 2007). The minimum standard deviation (Table 6) observed in the antimicrobial activity data justifies its use in QSAR studies.
Development of multi-target QSAR model
According to the above ot-QSAR models, one should use five different equations with different errors to predict the activity of a new compound against the five microbial species. The ot-QSAR models, which are almost in the whole literature, become impracticable, or at worse, too complicated to use when we have to predict to each compound the results for more than one target. However, very recently, the interest has become increased in the development of multi-target QSAR (mt-QSAR) models. In contrast to ot-QSAR, the mt-QSAR model is a single equation that considers the nature of molecular descriptors which are common and essential for describing the antibacterial and antifungal activity (Prado-Prado et al., 2008; Gonzalez-Diaz et al., 2008; Cruz-Monteagudo et al., 2007; Gonzalez-Diaz et al., 2007; Gonzalez-Diaz and Prado-Prado, 2008).
In the present study, we have attempted to develop three different types of mt-QSAR models: mt-QSAR model for describing the antibacterial activity of the synthesized compounds against S. aureus, B. subtilis, and E. coli,; mt-QSAR model for describing the antifungal activity of the synthesized derivatives against C. albicans and A. niger; and common mt-QSAR model for describing the antimicrobial (overall antibacterial and antifungal) activity of the synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives.
In order to develop mt-QSAR models, initially, we have calculated the average antibacterial, antifungal, and antimicrobial activity values of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives which are presented in Table 6. These average activity values were also correlated with the molecular descriptors of synthesized compounds (Table 9).
The mt-QSAR model for antibacterial activity displayed the importance of the third-order molecular connectivity topological index (3χ) in describing the antibacterial activity of synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives, represented as the model expressed by Eq. 6.
mt-QSAR model for antibacterial activity
The mt-QSAR model for antifungal activity demonstrated the importance of third-order molecular connectivity topological index (3χ) in describing the antifungal activity of the synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives represented by Eq. 7.
mt-QSAR model for antifungal activity
The model for antifungal activity developed by linear regression has got r value of 0.701, but, if we go for multiple linear regression analysis, the r value increased to 0.926, when we combined log P and valence third-order molecular connectivity topological index (3χv), and the model is represented by Eq. 8
mt-QSAR model for antifungal activity by MLR
Similarly, the overall antimicrobial activity of synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives is also governed by third-order molecular connectivity topological index (3χ), and represented in the model expressed by Eq. 9
mt-QSAR model for antimicrobial activity
Similarly, if we go for model generation by multiple linear regression analysis, the r value increased to 0.911 when we combined log P and valence third-order molecular connectivity topological index (3χv), and the model is represented by Eq. 10
mt-QSAR model for antimicrobial activity by MLR
The mt-QSAR models (Eqs. 6–10) for the antibacterial, antifungal, and overall antimicrobial activities of synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives depicted the fact that topological parameters, viz. third-order molecular connectivity topological index (3χ), govern all these activities. It was also observed that antifungal antimicrobial activity of the synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives are best explained by MLR models involving molecular descriptors log P and valence third-order molecular connectivity topological index (3χv). The developed mt-QSAR models were statistically valid as they had the high r and low residual values (Table 12). The low values of PE, LSE, LOFs and SEP and the high value of Q, (Table 11) revealed the statistical significance of the model described by Eqs. 6–10.
Conclusion
A series of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives (1–12) was synthesized and tested for their in vitro antimycobacterial activity against Mycobacterium tuberculosis, and the compounds with Cl, Br, NO2 groups and cycloheptylidene ring were found to be active ones. Compound 2 was found to be more active antimycobacterial agent than isoniazid, and compound 3 was also found to be equally active. The results of antiviral activity testing showed that none of the tested compounds was active at subtoxic concentrations. The anti-DNA virus activity of compounds 8 and 10 may likely be due to indirect cytostatic activity. The synthesized compounds were also screened for their antimicrobial potentials against S. aureus, B. subtilis, E. coli, C. albicans, and A. niger, and the results of antimicrobial activity indicated that compounds having Br, OCH3, and Cl groups were highly active antimicrobial agents with some of them exhibiting activities better than the standard compounds, norfloxacin and fluconazole. To understand the relationship between physicochemical parameters and antibacterial and antifungal activity of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives, QSAR investigation was performed by the development of one-target and multi-target models. The multi-target model was found to be effective in describing the antimicrobial activity of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives in comparison to the one-target models and indicated the importance of MLR models involving log P and valence third-order molecular connectivity topological index (3χv) in describing antifungal and overall antimicrobial activities, and the third-order molecular connectivity topological index (3χ) best describes the antibacterial potentials of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazide derivatives.
References
Bajaj S, Sambi SS, Madan AK (2005) Prediction of anti-inflammatory activity of N-arylanthranilic acids: computational approach using refined Zagreb Indices. Croat Chem Acta 78(2):165–174
Balaban AT (1982) Highly discriminating distance-based topological index. Chem Phys Lett 89:399–404
Bayrak H, Demirbas A, Demirbas N, Karaoglu SA (2009a) Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur J Med Chem 44:4362–4366
Bayrak H, Demirbas A, Karaoglu SA, Demirbas N (2009b) Synthesis of some new 1,2,4-triazoles, their mannich and schiff bases and evaluation of their antimicrobial activities. Eur J Med Chem 44:1057–1066
Boojamra CG, Mackman RL, Markevitch DY, Prasad V, Ray AS, Douglas J, Grant D, Kim CU, Cihlar T (2008) Synthesis and anti-HIV activity of GS-9148 (2′-Fd4AP), a novel nucleoside phosphonate HIV reverse transcriptase inhibitor. Bioorg Med Chem Lett 18:1120–1123
Cappucino JG, Sherman N (1999) Microbiology—a laboratory manual. Addison Wesley Longman Inc, California, p 263
Cruz-Monteagudo M, Gonzalez-Diaz H, Aguero-Chapin G, Santana L, Borges F, Dominguez ER, Podda G, Uriarte E (2007) Computational chemistry development of a unified free energy Markov model for the distribution of 1300 chemicals to 38 different environmental or biological systems. J Comput Chem 28(11):1909–1923
Dai J, Sun C, Han S, Wang L (1999) QSAR for polychlorinated compounds (PCOCs). I. Prediction of partition properties for PCOCs using quantum chemical parameters. Bull Environ Contam Toxicol 62:530–538
Emami S, Foroumadi A, Falahati M, Lotfali E, Rajabalian S, Ebrahimi FS, Shafiee A (2008) 2-Hydroxyphenacyl azoles and related azolium derivatives as antifungal agents. Bioorg Med Chem Lett 18:141–146
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1998) Vogel’s text book of practical organic chemistry. AddisonWesley Longman Inc, California, p 34
Furusjo E, Svenson A, Rahmberg M, Andersson M (2006) The importance of outlier detection and training set selection for reliable environmental QSAR predictions. Chemosphere 63:99–108
Golbraikh A, Tropsha A (2002) Beware of q 2!. J Mol Graphics Model 20:269–276
Gonzalez-Diaz H, Prado-Prado FJ (2008) Unified QSAR and network-based computational chemistry approach to antimicrobials, part 1: multispecies activity models for antifungals. J Comput Chem 29(4):656–667
Gonzalez-Diaz H, Vilar S, Santana L, Uriarte E (2007) Medicinal chemistry and bioinformatics—current trends in drugs discovery with networks topological indices. Curr Top Med Chem 7(10):1015–1029
Gonzalez-Diaz H, Gonzalez-Diaz Y, Santana L, Ubeira FM, Uriarte E (2008) Networks and connectivity indices. Proteomics 8(4):750–778
Guven OO, Erdogan T, Goker H, Yildiz S (2007) Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers. Bioorg Med Chem 17:2233–2236
Hansch C, Fujita T (1964) p-σ-π analysis. A method for the correlation of biological activity and chemical structure. J Am Chem Soc 86:1616–1626
Hansch C, Leo A, Unger SH, Kim KH, Nikaitani D, Lien EJ (1973) Aromatic substituent constants for structure–activity correlations. J Med Chem 16:1207–1216
Hatya SA, Aki-sener E, Tekiner-Gulbas B, Yildiz I, Temiz-Arpaci O, Yalcin I, Altanlar N (2006) Synthesis, antimicrobial activity and QSARs of new benzoxazine-3-ones. Eur J Med Chem 41:1398–1404
Hyperchem 6.0 (1993) Hypercube, Inc., Florida
Ivanciuc O, Ivanciuc T, Cabrol-Bass D (2002) QSAR for dihydrofolate reductase inhibitors with molecular graph structural descriptors. J Mol Str (Theochem) 582:39–51
Johnson K, Schultz PG (1994) Mechanistic studies of the oxidation of isoniazid by catalase-peroxidase from Mycobacterium tuberculosis. J Am Chem Soc 116:7425–7426
Judge V, Narang R, Sharma D, Narasimhan B, Kumar P (2010) Hansch analysis for the prediction of antimycobacterial activity of ofloxacin derivatives. Med Chem Res. doi:10.1007/s00044-010-9400-8.
Kamal A, Reddy KS, Ahmed SK, Khan MNA, Sinha RK, Yadav JS, Arora SK (2006) Antitubercular agents. Part 3. Benzothiadiazine as novel scaffold for antimycobacterium activity. Bioorg Med Chem 14:650–658
Khasnobis S, Escuyer VE, Chatterjee D (2002) Emerging therapeutic targets in tuberculosis: post genomic era. Expert Opin Ther Targets 6(1):21–40
Kier LB, Hall LH (1976) Molecular connectivity in chemistry and drug research. Academic Press, New York
Kim YM, Farrah S, Baney RH (2007) Structure–antimicrobial activity relationship for silanols, a new class of disinfectants, compared with alcohols and phenols. Int J Antimicrob Agents 29:217–222
Kucukguzel I, Tatar E, Kucukguzel SG, Rollas S, De Clercq E (2008) Synthesis of some novel thiourea derivatives obtained from 5-[(4-aminophenoxy)methyl]-4-alkyl/aryl-2,4-dihydro-3H–1,2,4-triazole-3-thiones and evaluation as antiviral/anti-HIV and anti-tuberculosis agents. Eur J Med Chem 43:381–392
Kumar A, Narasimhan B, Kumar D (2007) Synthesis, antimicrobial, and QSAR studies of substituted benzamides. Bioorg Med Chem 15:4113–4124
Kumar D, Judge V, Narang R, Sangwan S, De Clercq E, Balzarini J, Narasimhan B (2010a) Benzylidene/2-chlorobenzylidene hydrazides: synthesis, antimicrobial activity, QSAR studies and antiviral evaluation. Eur J Med Chem 45:2806–2816
Kumar P, Narasimhan B, Yogeeswari P, Sriram D (2010b) Synthesis and antitubercular activities of substituted benzoic acid N′-(substituted benzylidene/furan-2-ylmethylene)-N-(pyridine-3-carbonyl)-hydrazides. Eur J Med Chem 45:6085–6089
Lather V, Madan AK (2005) Topological models for the prediction of anti-HIV activity of dihydro (alkylthio) (napthylmethyl) oxopyrimidines. Bioorg Med Chem 13:1599–1604
Maccari R, Ottana R, Monforte F, Vigorita MG (2002) In vitro antimycobacterial activities of 2′-monosubstituted isonicotinohydrazides and their cyanoborane adducts. Antimicrob Agents Chemother 46(2):294–299
Mamolo MG, Zampieri D, Falagiani V, Vio L, Fermeglia M, Ferrone M, Pricl S, Banfi E, Scialino G (2004) Antifungal and antimycobacterial activity of new N1-[1-aryl-2-(1H-Imidazol-1-yl and 1H-1,2,4-triazol-1-yl)-ethylidene]- pyridine-2-carboxamidrazone derivatives: a combined experimental and computational approach. ARKIVOC v:231–250
Mandloi D, Joshi S, Khadikar PV, Khosla N (2005) QSAR study on the antibacterial activity of some sulfa drugs: building blockers of Mannich bases. Bioorg Med Chem Lett 15:405–411
Masunari A, Tavares LC (2007) A new class of nifuroxazide analogues: synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug resistant Staphylococcus aureus. Bioorg Med Chem Lett 15:4229–4236
Narasimhan B, Judge V, Narang R, Ohlan S, Ohlan R (2007) Quantitative structure–activity relationship studies for prediction of antimicrobial activity of synthesized 2,4-hexadienoic acid derivatives. Bioorg Med Chem Lett 17:5836–5845
National Committee for Clinical Laboratory Standards (1995) Antimycobacterial susceptibility testing for Mycobacterium tuberculosis. Proposed standard M24-T. National Committee for Clinical Laboratory Standards, Villanova, Pa
Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E (1988) Rapid and automated tetrazolium based colorimetric assay for detection of anti-HIV compounds. J Virol Methods 20:309–322
Pharmacopoeia of India (2007) Vol. I, Controller of Publications. Ministry of Health Department, Government of India, New Delhi, p 37
Pinheiro AAC, Borges RS, Santos SL, Alves CN (2004) A QSAR study of 8.O.4′-neolignans with antifungal activity. J Struct Mol (Theochem) 672:215–219
Prado-Prado FJ, Gonzalez-Diaz H, Vega OMDL, Ubeira FM, Chou KC (2008) First multi-tasking QSAR model for Input-Coded prediction, structural back-projection, and complex networks clustering of antiprotozoal compounds. Bioorg Med Chem 16(11):5871–5880
Randic M (1975) Characterization of molecular branching. J Am Chem Soc 97:6609–6615
Randic M (1993) Comparative regression analysis. Regressions based on a single descriptor. Croat Chem Acta 66:289–312
Rodriguez-Arguelles MC, Lopez-Silva EC, Sanmartin J, Pelagatti P, Zani F (2007) Copper complexes of imidazole-2-, pyrrole-2- and indol-3-carbaldehyde thiosemicarbazones: inhibitory activity against fungi and bacteria. J Inorg Biochem 101:138–147
Sahu KK, Ravichandran V, Mourya VK, Agrawal RK (2007) QSAR analysis of caffeoyl naphthalene sulfonamide derivatives as HIV-1 integrase inhibitors. Med Chem Res 15:418–430
Sbardella G, Mai A, Artico M, Setzu MG, Poni G, Colla PL (2004) New 6-nitroquinolones: synthesis and antimicrobial activities. IL Farmaco 59:463–471
Shahar Yar M, Siddiqui AA, Ali MA (2007) Synthesis and antimycobacterial activity of novel heterocycles. J Serb Chem Soc 72(1):5–11
Sharma P, Rane N, Gurram VK (2004) Synthesis and QSAR studies of pyrimido[4,5-d] pyrimidine-2,5-dione derivatives as potential antimicrobial agents. Bioorg Med Chem Lett 14:4185–4190
Sharma P, Kumar A, Sharma M (2006) Synthesis and QSAR studies on 5-[2-(2-methylprop-1-enyl)-1Hbenzimidazol-1yl]-4,6-diphenyl-pyrimidin-2-(5H)-thione derivatives as antibacterial. Eur J Med Chem 41:833–840
Shoeb HA, Bowman BU Jr, Ottolenghi AC, Merola AJ (1985) Peroxidase mediated oxidation of isoniazid. Antimicrob Agents Chemother 27:399–403
Sortino M, Delgado P, Jaurez S, Quiroga J, Abonia R, Insuasey B, Rodero MN, Garibotto FM, Enriz RD, Zacchino SA (2007) Synnthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg Med Chem Lett 15:484–494
SPSS for Windows, version 10.05 (1999) SPSS Inc., Bangalore, India
Sriram D, Yogeeswari P, Dhakla P, Senthilkumar P, Banerjee D (2007) N-Hydroxythiosemicarbazones: synthesis and antitubercular activity. Bioorg Med Chem Lett 17:1888–1891
Tripathi RP, Saxena N, Tiwari VK, Verma SS, Chaturvedi V, Manju YK, Srivastva AK, Gaikwad A, Sinha S (2006) Synthesis and antitubercular activity of substituted phenylmethyl- and pyridylmethyl amines. Bioorg Med Chem 4:8186–8196
TSAR 3D Version 3.3 (2000), Oxford Molecular Limited
Wiener H (1947) Structural determination of paraffin boiling points. J Am Chem Soc 69:17–20
Zhang P, Heym B, Allen B, Young D, Cole ST (1992) The catalase-peroxidase gene and isoniazid resistance to Mycobacterium tuberculosis. Nature 358:591–593
Acknowledgments
One of the authors (VJ) is thankful to the Department of Technical Education, Government of Haryana (India) for providing fellowship to carry out the research project. The authors would like to thank Mrs. Leentje Persoons, Mrs. Frieda De Meyer, Mrs. Kristien Erven, Mr. Kris Uyttersprot, Mrs. Anita Camps, Mrs. Lies Van den Heurck, Mr. Steven Carmans, and Mrs. Leen Ingels, Rega Institute for Medical Research, Belgium for their excellent technical assistance in the evaluation of antiviral activity. Financial support provided by the GOA (no. 10/014) of the K.U. Leuven is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Judge, V., Narasimhan, B., Ahuja, M. et al. Synthesis, antimycobacterial, antiviral, antimicrobial activities, and QSAR studies of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides. Med Chem Res 21, 1935–1952 (2012). https://doi.org/10.1007/s00044-011-9705-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9705-2