Abstract
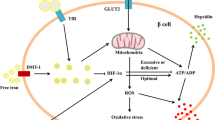
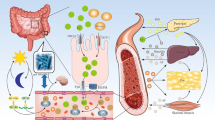
Diabetes mellitus is frequently associated with iron overload conditions, such as primary and secondary hemochromatosis. Conversely, patients affected by type 2 diabetes mellitus (T2DM) show elevated ferritin levels, a biomarker for increased body iron stores. Despite these documented associations between dysregulated iron metabolism and T2DM, the underlying mechanisms are poorly understood. Here, we show that T2DM patients have reduced serum levels of hepcidin, the iron-regulated hormone that maintains systemic iron homeostasis. Consistent with this finding, we also observed an increase in circulating iron and ferritin levels. Our analysis of db/db mice demonstrates that this model recapitulates the systemic alterations observed in patients. Interestingly, db/db mice show an overall hepatic iron deficiency despite unaltered expression of ferritin and the iron importer TfR1. In addition, the liver correctly senses increased circulating iron levels by activating the BMP/SMAD signaling pathway even though hepcidin expression is decreased. We show that increased AKT phosphorylation may override active BMP/SMAD signaling and decrease hepcidin expression in 10-week old db/db mice. We conclude that the metabolic alterations occurring in T2DM impact on the regulation of iron homeostasis on multiple levels. As a result, metabolic perturbations induce an “iron resistance” phenotype, whereby signals that translate increased circulating iron levels into hepcidin production, are dysregulated.
Key messages
-
T2DM patients show increased circulating iron levels.
-
T2DM is associated with inappropriately low hepcidin levels.
-
Metabolic alterations in T2DM induce an “iron resistance” phenotype.




Similar content being viewed by others
References
Roglic G, World Health Organization (2016) Global report on diabetes. World Health Organization, Geneva
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Altamura S, Muckenthaler MU (2009) Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J Alzheimers Dis 16:879–895
Niederau C, Fischer R, Purschel A, Stremmel W, Haussinger D, Strohmeyer G (1996) Long-term survival in patients with hereditary hemochromatosis. Gastroenterology 110:1107–1119
Fargion S, Mandelli C, Piperno A, Cesana B, Fracanzani AL, Fraquelli M, Bianchi PA, Fiorelli G, Conte D (1992) Survival and prognostic factors in 212 Italian patients with genetic hemochromatosis. Hepatology 15:655–659
Adams PC, Speechley M, Kertesz AE (1991) Long-term survival analysis in hereditary hemochromatosis. Gastroenterology 101:368–372
Niederau C (1999) Diabetes mellitus in hemochromatosis. Z Gastroenterol Suppl 1:22–32
Canturk Z, Cetinarslan B, Tarkun I, Canturk NZ (2003) Serum ferritin levels in poorly- and well-controlled diabetes mellitus. Endocr Res 29:299–306
Aregbesola A, Voutilainen S, Virtanen JK, Mursu J, Tuomainen TP (2013) Body iron stores and the risk of type 2 diabetes in middle-aged men. Eur J Endocrinol 169:247–253
Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113:1271–1276
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
Sam AH, Busbridge M, Amin A, Webber L, White D, Franks S, Martin NM, Sleeth M, Ismail NA, Daud NM et al (2013) Hepcidin levels in diabetes mellitus and polycystic ovary syndrome. Diabet Med 30:1495–1499
Guo X, Zhou D, An P, Wu Q, Wang H, Wu A, Mu M, Zhang D, Zhang Z, Wang H et al (2013) Associations between serum hepcidin, ferritin and Hb concentrations and type 2 diabetes risks in a Han Chinese population. Br J Nutr 110:2180–2185
Jiang F, Sun ZZ, Tang YT, Xu C, Jiao XY (2011) Hepcidin expression and iron parameters change in type 2 diabetic patients. Diabetes Res Clin Pract 93:43–48
Andrews M, Soto N, Arredondo-Olguin M (2015) Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition 31:51–57
Suarez-Ortegon MF, Moreno M, Arbelaez A, Xifra G, Mosquera M, Moreno-Navarrete JM, Aguilar-de Plata C, Esteve E, Ricart W, Fernandez-Real JM (2015) Circulating hepcidin in type 2 diabetes: a multivariate analysis and double blind evaluation of metformin effects. Mol Nutr Food Res 59:2460–2470
Brückel J, Kerner W (2006) Definition, klassifikation und diagnostik des diabetes mellitus. Diabetologie und Stoffwechsel 1:177–180
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Rametta R, Dongiovanni P, Pelusi S, Francione P, Iuculano F, Borroni V, Fatta E, Castagna A, Girelli D, Fargion S et al (2016) Hepcidin resistance in dysmetabolic iron overload. Liver Int 36:1540–1548
Rabbani N, Thornalley PJ (2014) Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat Protoc 9:1969–1979
Ejdesjo A, Brings S, Fleming T, Fred RG, Nawroth PP, Eriksson UJ (2016) Receptor for advanced glycation end products (RAGE) knockout reduces fetal dysmorphogenesis in murine diabetic pregnancy. Reprod Toxicol 62:62–70
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Torrance JD, Bothwell TH (1968) A simple technique for measuring storage iron concentrations in formalinised liver samples. The South African journal of medical sciences 33:9–11
Harrison-Findik DD (2010) Gender-related variations in iron metabolism and liver diseases. World J Hepatol 2:302–310
Jacobs A (1976) Sex differences in iron absorption. Proc Nutr Soc 35:159–162
Cook JD, Finch CA, Smith NJ (1976) Evaluation of the iron status of a population. Blood 48:449–455
Hummel KP, Dickie MM, Coleman DL (1966) Diabetes: a new mutation in the mouse. Science 153:1127–1128
Muckenthaler MU (2008) Fine tuning of hepcidin expression by positive and negative regulators. Cell Metab 8:1–3
Muckenthaler MU, Galy B, Hentze MW (2008) Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr 28:197–213
Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A (2009) ER stress controls iron metabolism through induction of hepcidin. Science 325:877–880
Vecchi C, Montosi G, Garuti C, Corradini E, Sabelli M, Canali S, Pietrangelo A (2014) Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 146:1060–1069
Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L et al (2005) A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab 2:399–409
Steinbicker AU, Sachidanandan C, Vonner AJ, Yusuf RZ, Deng DY, Lai CS, Rauwerdink KM, Winn JC, Saez B, Cook CM et al (2011) Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood 117:4915–4923
Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI (1992) Increased rate of gluconeogenesis in type II diabetes mellitus: a 13C nuclear magnetic resonance study. J Clin Invest 90:1323–1327
Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK et al (2001) Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138
Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P et al (2001) CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183
Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M (1994) Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem 269:3568–3573
Frevert EU, Bjorbaek C, Venable CL, Keller SR, Kahn BB (1998) Targeting of constitutively active phosphoinositide 3-kinase to GLUT4-containing vesicles in 3T3-L1 adipocytes. J Biol Chem 273:25480–25487
Mleczko-Sanecka K, Roche F, da Silva AR, Call D, D’Alessio F, Ragab A, Lapinski PE, Ummanni R, Korf U, Oakes C et al (2014) Unbiased RNAi screen for hepcidin regulators links hepcidin suppression to proliferative Ras/RAF and nutrient-dependent mTOR signaling. Blood 123:1574–1585
Goodnough JB, Ramos E, Nemeth E, Ganz T (2012) Inhibition of hepcidin transcription by growth factors. Hepatology 56:291–299
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297
Huth C, Beuerle S, Zierer A, Heier M, Herder C, Kaiser T, Koenig W, Kronenberg F, Oexle K, Rathmann W et al (2015) Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. Eur J Endocrinol 173:643–653
Alam F, Fatima F, Orakzai S, Iqbal N, Fatima SS (2014) Elevated levels of ferritin and hs-CRP in type 2 diabetes. J Pak Med Assoc 64:1389–1391
Kunutsor SK, Apekey TA, Walley J, Kain K (2013) Ferritin levels and risk of type 2 diabetes mellitus: an updated systematic review and meta-analysis of prospective evidence. Diabetes Metab Res Rev 29:308–318
Wang H, Li H, Jiang X, Shi W, Shen Z, Li M (2014) Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes 63:1506–1518
Segev Y, Eshet R, Yakir O, Haim N, Phillip M, Landau D (2007) Systemic and renal growth hormone-IGF1 axis involvement in a mouse model of type 2 diabetes. Diabetologia 50:1327–1334
Peslova G, Petrak J, Kuzelova K, Hrdy I, Halada P, Kuchel PW, Soe-Lin S, Ponka P, Sutak R, Becker E et al (2009) Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood 113:6225–6236
Senates E, Yilmaz Y, Colak Y, Ozturk O, Altunoz ME, Kurt R, Ozkara S, Aksaray S, Tuncer I, Ovunc AO (2011) Serum levels of hepcidin in patients with biopsy-proven nonalcoholic fatty liver disease. Metab Syndr Relat Disord 9:287–290
Demircioglu F, Gorunmez G, Dagistan E, Goksugur SB, Bekdas M, Tosun M, Kizildag B, Kismet E (2014) Serum hepcidin levels and iron metabolism in obese children with and without fatty liver: case-control study. Eur J Pediatr 173:947–951
Dongiovanni P, Fracanzani AL, Fargion S, Valenti L (2011) Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol 55:920–932
Senyilmaz D, Virtue S, Xu X, Tan CY, Griffin JL, Miller AK, Vidal-Puig A, Teleman AA (2015) Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature 525:124–128
Wang B, Chandrasekera PC, Pippin JJ (2014) Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 10:131–145
Acknowledgements
We would like to thank the Nikon center of the University of Heidelberg and the Institute of Pathology of the University Hospital of Heidelberg for granting us access to their facilities. We are particularly thankful to Regina Kessler and Thomas Fleming for their support in this project. We would also like to thank Natalie K. Horvat for the critical evaluation of the manuscript.
Funding
This work is supported by the Deutsche Forschungsgemeinschaft (SFB1118).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethic committees of the University of Heidelberg approved this study (Decision No. 204/2004 and 400/2010) according to the Declaration of Helsinki. All patients entered the study according to the guidelines of the local ethics committees following written informed consent to participate.
Animal experiments were approved by and conducted in compliance with the guidelines of the University of Heidelberg (Project T-94/15).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 28 kb)
Rights and permissions
About this article
Cite this article
Altamura, S., Kopf, S., Schmidt, J. et al. Uncoupled iron homeostasis in type 2 diabetes mellitus. J Mol Med 95, 1387–1398 (2017). https://doi.org/10.1007/s00109-017-1596-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1596-3