Abstract
An investigation to identify a sex or aggregation pheromone of Sitona discoideus Gyllenhål (Coleoptera: Curculionidae) is presented. Antenna flicking and attraction behaviors evoked by conspecifics of both sexes were recorded in arena bioassays, where attraction of females to males was observed. Air entrainment of both males and females was conducted in separate chambers. Gas chromatographic–mass spectrometric analysis of headspace volatiles revealed that two male-specific compounds, 4-methyl-3,5-heptanedione (major) and (4S,5S)-5-hydroxy-4-methyl-3-heptanone (minor), were emitted during the autumnal post-aestivatory flight period. The stereoisomers of the minor component were separated by enantioselective gas chromatography and their absolute configurations assigned by NMR (diastereomers) and the known preference of enantioselective transesterification reactions catalyzed by Candida antarctica lipase B. Electroantennogram and single sensillum recording studies indicate that 4-methyl-3,5-heptanedione as well as all individual stereoisomers of 5-hydroxy-4-methyl-3-heptanone are detected by the antennae of male and female S. discoideus. Further, single sensillum recordings suggest that both sexes of S. discoideus have specialized olfactory receptor neurons (ORNs) for detecting 4-methyl-3,5-heptanedione and different populations of stereoselective ORNs for detecting the stereoisomers of 5-hydroxy-4-methyl-3-heptanone. Some of these stereoselective ORNs appear to be sex-specific in S. discoideus.
Similar content being viewed by others
Introduction
The Mediterranean pest of Medicago spp. (L.), the lucerne weevil, Sitona discoideus (Gyllenhål) (Coleoptera: Curculionidae) was accidentally introduced to New Zealand in the early 1970s (Esson 1975), probably via Australia where the weevil had been established since the 1950s (Chadwick 1960). In New Zealand, S. discoideus quickly became established in lucerne (Medicago sativa) crops throughout the country. S. discoideus was found to be a serious pest of lucerne, with significant reductions in plant productivity, principally not only through larval mining of nitrogen-fixing root nodules and general herbivory on the root system but also through adult defoliation of leaves (Kain and Trough 1982; Goldson and French 1983; Goldson et al. 1985).
The pest exhibits univoltine, aestivatory seasonality in Canterbury and Otago, with adults of each generation appearing in late December (NZ summer) vigorously feeding on lucerne foliage for 2–4 weeks, before commencing summer aestivation in late January–February (Goldson et al. 1984). Sexually immature weevils remain at aestivation sites such as hedgerows, under stones, and at the base of trees and fence posts, for a period of 6–8 weeks. Initially during this period, their indirect flight muscles degenerate (Frampton 1987), but by late March to mid-May (NZ autumn), the muscles have been regenerated, and maturation of the reproductive organs commences. The weevils then return to the lucerne fields, in the autumnal post-aestivatory flight, for feeding and mating (Goldson et al. 1984). The adults stay in the lucerne during winter, with egg-laying activity occurring primarily in April–May and October–November. Age-related mortality commences in October (Goldson et al. 1984).
The introduction of a biological control agent Microctonus aethiopoides Loan (Hymenoptera: Braconidae) did result in a reduction of the damage by S. discoideus in New Zealand (Goldson et al. 1990), but farmer reports of yield losses in newly established lucerne crops (McNeill, M.R., personal communication) meant that there was a requirement to manage the pest, especially in the first 1–2 years following crop establishment. For this reason, a monitoring tool for S. discoideus would facilitate the assessment of pest numbers and planning of insecticide treatments to control the weevil before significant egg-laying activity could occur, and damaging larval populations could ensue. To this end, initial replicated field trials were undertaken using boll weevil traps baited with 4-methyl-3,5-heptanedione similar to the system used for Sitona lineatus (Blight et al. 1991). However, while there was evidence that on occasions, significantly more S. discoideus were caught in the traps baited with 4-methyl-3,5-heptanedione than the control, results were variable (Wee, S.L. and McNeill, M.R., personal communication). The variability could be attributed either to the efficacy of the compound as an attractant to the weevils or to unsuitable trap design in terms of compound release rate or the ability of the weevils to find and enter the trap. Therefore, after initial field trials, research commenced to identify a pheromone that could be used in the early detection of adult S. discoideus.
To the best of our knowledge, the first and only evidence of an aggregation pheromone in a Sitona species, i.e., 4-methyl-3,5-heptanedione, was reported by Blight and colleagues in the pea leaf weevil, S. lineatus (L.) (Blight et al. 1984, 1991). Later, Nielsen and Jensen (1993) attempted to monitor the spring dispersal of S. lineatus using pheromone traps. Toth and colleagues (1998) reported that 4-methyl-3,5-heptanedione was a general attractant to other members of the Sitona genus including S. macularius and S. humeralis.
Here, we describe the identification of male-produced volatiles and the induced behavior in the lucerne weevil, S. discoideus. The effects of these volatiles were monitored on both sexes, and corresponding selective responses in different receptor neurons are reported. Our results could turn out to be useful in future pest control and management of S. discoideus.
Materials and methods
Insects
Adult S. discoideus weevils were collected from field populations in lucerne paddocks in Lincoln (S43.6221, E172.4709) and Darfield (S43.4882, E172.1452), Canterbury, New Zealand, using a modified commercial blower vac (Echo ES-2400, 24 cc; Kioritz Corporation, Tokyo). The weevils used in bioassays were collected in autumn, having returned to the lucerne paddocks following the post-aestivatory flights. Therefore, they were from the December-emerged population and of unknown mating status. The weevils were sexed by examination under a stereomicroscope (×10 magnification) after being anesthetized with carbon dioxide. Individuals were separated by gender based on the shape of the eighth abdominal ventrite. For females, the posterior margin of the ventrite is uniformly rounded, whereas for males, it is truncated with the pygidium narrowly exposed. A subsample was also dissected to determine reproductive maturity. Before trials, males and females were maintained separately on fresh lucerne leaves in vented plastic containers (220 × 130 × 75 mm) at 15 ± 2 °C and 68 % RH under a 16:8-h light/dark regime.
Laboratory bioassays
A Petri dish assay was used to determine the behavioral response of a single test weevil (male or female), placed in the outer 50 % area of the 90 × 20-mm Petri dish, to three male or female weevils confined in a central 2-cm-diameter stainless steel gauze ring for 10 min. An anti-stick compound (Whitford Pte Ltd., Singapore) was applied to the rim of the dish to prevent the test weevil from escaping. The parameters recorded were (1) the location of the test weevils over time (i.e., percentage of total time spent in the equal sized center or edge portions of the arena in a 10-min assay) and (2) the antennation behavior of responding female weevils (absence or presence of continuous bouts of vigorous antennal flicking in a 5-min assessment). The assay was replicated eight times (n = 8) for each attraction source (three males or three females) and test subject (individual males or females). In the second experiment, similar bioassay procedures for location and antennal response were employed except that the female weevils were confined in isolation for a period of 3, 7, 10, and 14 days prior to the assays. The percentage of time spent in the center or edge zones of the assay arena, in response to three male or female weevils in the central ring, was recorded (n = 10).
Chemical analyses
Gas chromatographic–mass spectrometric (GC-MS) analyses were conducted using a Varian CP-3800 gas chromatograph equipped with a VF-5MS column [30 m × 0.25 mm internal diameter (i.d.) × 0.25 μm thickness] and connected to an ion trap Varian Saturn 2200 MS with an electron impact of 70 eV and source temperature of 250 °C. Injection volume was 1 μl in splitless mode. The carrier gas was helium, and the oven temperature was programmed to increase from 40 °C (5 min hold) to 250 °C with 5 °C/min. Chemical identity of volatiles from all treatments was confirmed by comparing the retention times and mass fragment patterns with synthetic compounds. For enantioselective analyses, a CycloSil-B column (30 m × 0.25 mm i.d. × 0.25 μm film; J & W Scientific) and an isothermal GC column temperature of 89 °C were used. All other conditions were identical.
Chemicals
Chemicals were obtained from Sigma-Aldrich, Germany or USA except 4-methyl-3,5-heptanedione and 5-hydroxy-4-methyl-3-heptanone, which were synthesized as described in the Supplementary material. Candida antarctica lipase B (CALB) was obtained from Sigma-Aldrich, Germany, as a macroporous acrylic resin expressed in Aspergillus niger. Before use in electrophysiological experiments, all compounds were confirmed to have a chemical purity of min 95 % and a stereoisomeric purity of min 95 %. Preparative column chromatography was applied to purify and separate diastereomers when needed.
Acetylation of 5-hydroxy-4-methyl-3-heptanone using CALB
5-Hydroxy-4-methyl-3-heptanone [100 mg, 0.69 mmol (sum of isomers), 60 % syn] was mixed with vinyl acetate (500 μl, 6.25 mmol), and C. antarctica lipase B (50 mg) was added. The mixture was incubated at 25 °C/120 rpm in a shaking water bath, and the progress of the reaction was monitored by enantioselective GC. After 6 days, the reaction mixture was quenched by removal of the enzyme by filtration. The proportions of isomers at the start of the reaction are shown in Fig. 3, and the product composition of the remaining alcohols and acetates formed is shown in Fig. 5.
Headspace sampling of weevils
Headspace sampling was conducted for male and female weevils using the dynamic headspace sampling method in an air entrainment system. Reproductive condition was classified as intermediate to mature development with the majority of females mated. In the experiment, ca. 40 adult weevils of each sex were placed in two separate chambers (1 l) and with a third chamber as control. To minimize desiccation of weevils, eight dental rolls presoaked in tap water were also placed in each of the chambers. Volatiles released were actively adsorbed onto a Tenax cartridge (100 mg, GR 35/60; Grace Davison Discovery Science, Deerfield, IL, USA) for 24 h at an air speed of 3 l/min. Chemicals from the Tenax cartridges were extracted with diethyl ether (800 μl), and each of the eluates was concentrated to approximately 10 μl under a gentle argon stream. Aliquots of 1 μl were subjected to GC-MS analysis.
Electroantennogram and single sensillum recordings
Electroantennogram (EAG) and single sensillum responses to 4-methyl-3,5-heptanedione and four isomers of 5-hydroxy-4-methyl-3-heptanone were recorded in S. discoideus. For the EAG and single sensillum recording (SSR) experiments, a weevil was mounted, with its ventral side up, on a plasticine block with U-shaped copper wires, and each antenna was restrained from movement by pinning the antenna with fine U-shaped copper wires. A fine-tip glass electrode filled with 0.1 M KCl solution was introduced into the thorax to serve as a reference electrode. For EAG recordings, a glass capillary (0.86 mm ID, 3 cm long; A-M Systems, Inc., Everett, USA) filled with an electroconductive gel (Spectra®; Parker Laboratories, Inc., Fairfield, NJ, USA) served as the recording electrode. It was brought into contact with the tip of the antenna after removal of the distal part of the antenna. An Ag-AgCl wire was used to maintain electrical continuity between the electrodes and a high input impedance head-stage preamplifier (Universal AC/DC probe, Syntech®; The Netherlands). For SSR, the fine tip of an electrochemically sharpened tungsten rod (tip diameter of <0.1 μm) was introduced into the basal area of an antennal sensillum to ensure a stable electrical contact, using a micromanipulator. The EAG and SSR signals from the preamplifier were further amplified and processed with a PC-based signal processing system (IDAC 4, Syntech®; The Netherlands). Solutions (1 or 10 μg/μl) of each test compound were prepared in hexane. A 10 μl aliquot of each solution was applied onto a piece (5 × 30 mm) of filter paper (Whatman® no. 1; USA), and the filter paper strip was inserted into a glass Pasteur pipette (150 mm long; Volac). The tip of the pipette was inserted into a small hole of the main airflow tube (10 mm diameter) where a continuous, charcoal-filtered, and humidified airflow (1 l/min) was blown over the antennal preparation. A 0.1-s puff of charcoal-filtered airflow (10 ml/s) was injected through the large end of the Pasteur pipette odor cartridge for stimulation, using an electronic airflow controller (CS-55, Syntech®; The Netherlands). The antenna was stimulated by applying the test compounds in random order. A minimum of 1-min time interval was maintained between stimulations. For EAG, absolute EAG amplitudes (maximum depolarization) were used for data analysis. The EAG data were subjected to analysis of variance followed by Fisher’s LSD analysis. For SSR, the increase in the number of action potentials after odor stimulation was regarded as a response to the stimuli.
Electroantennogram dose–responses to volatiles emitted by S. discoideus
Excised antennae from adult male and female S. discoideus were used to determine EAG dose–responses for 4-methyl-3,5-heptanedione and all stereoisomers of 5-hydroxy-4-methyl-3-heptanone. The stimuli were prepared as serial dilutions with loadings of 0.1, 1.0, 10.0, 100.0, and 1,000.0 μg, using hexane as the solvent. Test solutions (20 μl) were applied to a strip of filter paper (10 × 30 mm), and after solvent evaporation, the filter paper strip was placed in a Pasteur pipette containing an odor cartridge. The antenna was continuously ventilated with a stream of charcoal-purified and humidified air (0.5 m/s) that passed through the right sleeve of a glass Y-tube (5 mm i.d; 40 mm long) with the main sleeve positioned 1.5 cm from the antenna. Stimuli were applied by injecting 1 ml of air from the odor cartridge impregnated with test compound as 1-s puffs into the continuous airstream through the left sleeve of the glass Y-tube. Odor stimulations were controlled by a Syntech stimulus controller (CS-05) operated by a foot switch.
The test chemicals were applied from the lowest to the highest concentrations to minimize antennal habituation. Stimulation intervals of minimum 1 min were allowed between stimuli. To compensate for antennal deterioration over time, linalool was used as a reference compound (10 ng/20 μl), which was puffed before and after stimulation of every two test compounds. EAG amplitudes were then normalized by dividing the amplitude of the EAG generated from the test compounds by that of the linalool standard. Each stimulus was tested on the antennae of six different weevils.
Results
Behavioral assays
The first Petri dish assay showed that male and female S. discoideus produced significantly different responses in tests with groups of three weevils of either sex in the central cylinder as stimuli. Females spent significantly more time in the central area of the dish when three males rather than three females were used as the central lure (t = −3.20; P = 0.006; degrees of freedom (df) = 14). Males, in contrast, showed no significant difference in behavior to either males or females (t = 0.20; P = 0.847; df = 14). In female weevils, the time spent in vigorous antennation was increased in the presence of males (~2.5-fold, from 12 to 31 % of time available) compared with the inactive antennal response dominant among females in the presence of female weevils. After isolation, females spent 16 % (±2.7 % s.e.) of the assay time antennating to females, compared with 39 % (±2.6 %) of time spent antennating to males. The time females spent in the center of the arena in the presence of three males as a central lure increased significantly when tested after 3, 7, 10, and 14 days of isolation (Fig. 1, F 3,35 = 13.71; P < 0.0001), but there was no difference when three females were in the center (F 3,32 = 2.64; P = 0.066). Antennation behavior in females in response to males was also increased with isolation in a similar way (F 3,35 = 6.72; P = 0.001) (data not shown), while males did not show this antennation.
Headspace sampling of weevils
Air entrainment of male and female S. discoideus followed by GC-MS analysis confirmed that both 4-methyl-3,5-heptanedione and 5-hydroxy-4-methyl-3-heptanone were exclusively emitted by the males. The dione was detected in >30 times higher amounts than the hydroxy ketone in the headspace above sexually mature male weevils.
Analytical separation and assignment of the GC retention order of the diastereomers of 5-hydroxy-4-methyl-3-heptanone
A synthetic mixture of the four stereoisomers of 5-hydroxy-4-methyl-3-heptanone could easily be prepared (Fig. 2). At this stage of the project, all individual isomers of this compound were not available. An analytical protocol was therefore developed, where all stereoisomers of the synthetic mixture, including the natural product, could be unambiguously assigned.
It is known that condensation of propanal with 3-pentanone yields a mixture of syn- and anti-isomers of 5-hydroxy-4-methyl-3-heptanone (Fig. 2) in a 3:2 syn/anti ratio, and that the two pairs of diastereomers have characteristic differences in their chemical shifts in 13C-NMR (Heathcock et al. 1979). Baseline separation of all four stereoisomers was obtained when our synthetic mixture was analyzed using an enantioselective column (Fig. 3). The two syn-isomers could be distinguished from the two later eluting anti-isomers. By comparison with the air entrainment extract from the male weevils analyzed with the same column, it could be concluded that only one of the anti-isomers of 5-hydroxy-4-methyl-3-heptanone was produced by the male weevils.
Assignment of the absolute stereochemistry and GC elution order of the four isomers of 5-hydroxy-4-methyl-3-heptanone
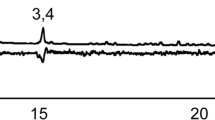
Certain commercially available lipases react enantioselectively with secondary alcohols in a predictable manner (Kazlauskas et al. 1991). In order to assign all stereoisomers of 5-hydroxy-4-methyl-3-heptanone, the two 5R alcohols were selectively acylated in the mixture by the lipase C. antarctica B (Fig. 4). When CALB was used as the catalyst and vinyl acetate as the acyl donor in the transesterification reaction, acetates were only formed from the hydroxy ketones eluted as the second and third peaks (Fig. 5). The second eluting compound reacted slightly faster than the third, which is in accordance with the known β-selectivity for this enzyme (Unelius et al. 1998).
Gas chromatographic trace on an enantioselective column (CycloSil-B) showing the product composition after selective acylation of 5R alcohols of 5-hydroxy-4-methyl-3-heptanone using the lipase C. antarctica B. The late eluting peaks are acetates of the (4S,5R)- and (4R,5R)-isomers [(4S,5R)-OAc, retention time (RT) = 36.22 min); (4R,5R)-OAc, RT = 37.22 min]
As the second and third eluting compounds were both capable of reacting with the enzyme, i.e., had 5R configuration, it was clear that the elution order was (4R,5S), (4S,5R), (4R,5R), and (4S,5S). The α values for the four stereoisomers of 5-hydroxy-4-methyl-3-heptanone were α syn 4R,5S /syn 4S,5R = 1.06, α syn 4S,5R /anti 4R,5R = 1.02, and α anti 4R,5R /anti 4S,5S = 1.14.
The retention times were for the four stereoisomers of 5-hydroxy-4-methyl-3-heptanone (4R,5S):29.0 min, (4S,5R):30.8 min, (4R,5R):31.9 min, and (4S,5S):36.5 min, applying an isothermal column temperature of 89 °C.
Assignment of the absolute stereochemistry of the isomer of 5-hydroxy-4-methyl-3-heptanone released by male lucerne weevils
Injection and analyses of the extracts obtained from air entrainment of male and female lucerne weevils in conjunction with injection of reference compounds revealed that (within the detection limits of our system) the females emit neither 4‐methyl‐3,5‐heptanedione nor 5-hydroxy-4-methyl-3-heptanone, and that only the (4S,5S)-isomer of 5-hydroxy-4-methyl-3-heptanone is produced by the male weevils (Fig. 6).
Gas chromatographic trace on an enantioselective column (CycloSil-B, SIM mode, m/z 126). Top trace indicates volatiles released by female weevils; middle trace, volatiles released by male weevils; and lower trace, in elution order: synthetic 4-methyl-3,5-heptanedione, its enol tautomer, and the four stereoisomers of 5-hydroxy-4-methyl-3-heptanone (the last peak is the (4S,5S)-isomer)
The stereoselective syntheses of the four stereoisomers in isomerically pure form have now been described and our initial assignment positively confirmed (Bohman and Unelius 2009).
Electroantennogram
Our initial EAG studies showed that the antennae of both sexes of the lucerne weevil responded to 4-methyl-3,5-heptanedione and the 3:2 syn/anti mixture of all isomers of 5-hydroxy-4-methyl-3-heptanone. The two antennally active stimuli exhibited similar EAG dose–responses between males and females, with a rapid increase in EAG responses when the load exceeded 100 μg (Fig. 7). There was no significant difference in the dose–response between the sexes.
Further EAG tests of both male and female S. discoideus showed significant EAG responses to 4-methyl-3,5-heptanedione and all individual stereoisomers of 5-hydroxy-4-methyl-3-heptanone at a 100-μg dose (Fig. 8). In these EAG experiments, 4-methyl-3,5-heptanedione elicited the largest EAG responses in both sexes. Among 5-hydroxy-4-methyl-3-heptanones, the (4S,5S)-isomer elicited slightly stronger EAG responses in males, in contrast to females, which showed similar EAG responses to all four isomers.
Electroantennogram responses (mean ± SE) of female and male S. discoideus to five putative pheromone components: 4-methyl-3,5-heptanedione (dione) and the four stereoisomers of 5-hydroxy-4-methyl-3-heptanone (100 μg of each compound, n = 24). Different letters above the bars indicate significant differences by Tukey’s HSD test (α = 0.05)
Single sensillum recordings
Single sensillum recording results showed that different types of olfactory receptor neurons (ORNs) for these compounds were present in both male and female S. discoideus. At least four different types of olfactory sensilla were identified in S. discoideus based on response profiles (Fig. 9). Both male and female S. discoideus appeared to have ORNs specialized for 4-methyl-3,5-heptanedione, where the small spike generating ORN in sensilla type A in male and the large spike generating ORN in sensilla type C in female S. discoideus responded exclusively to the heptanedione. It was evident that both male and female S. discoideus contained different ORN populations responding to (4S,5S)-5-hydroxy-4-methyl-3-heptanone and its stereoisomers, and some of these ORNs showed stereoselective responses (Fig. 9). For example, the small spike ORN in sensilla type B in male S. discoideus responded only to the (4R,5R)-isomer, while the large spike ORN in the same sensilla responded to (4S,5S)- and (4S,5R)-isomers. In type B sensilla, it was unclear if the responses to 4-methyl-3,5-heptanedione were elicited by the same ORN responding to (4S,5S)- and (4S,5R)-isomers, since no difference in the spike sizes could be found. No ORNs in the sensilla type B responded to the (4R,5S)-isomer. It was also unclear if more than one type of ORN populations for the stereoisomers existed in the two other types (type A in male and type D in female) of sensilla, since the spike sizes were not distinguishable between different responses.
Responses of olfactory receptor neurons in male (two left columns) and female (two right columns) S. discoideus antennal sensilla to 10 μg of five potential pheromone compounds [dione 4-methyl-3,5-heptanedione; S,S (4S,5S)-5-hydroxy-4-methyl-3-heptanone; S,R (4S,5R)-5-hydroxy-4-methyl-3-heptanone; R,S (4R,5S)-5-hydroxy-4-methyl-3-heptanone; and R,R (4R,5R)-5-hydroxy-4-methyl-3-heptanone] and solvent control. Arrows indicate the onset of stimulation for 0.1 s
Discussion
Several Sitona species appear to use the same major component, 4-methyl-3,5-heptanedione, as an aggregation pheromone, although investigations have not been extensive (Toth et al. 1998). It is presumed that variations in blends may make it possible for weevils to differentiate between species and thereby prevent cross-species attraction or hybridization that might lead to sterility. Our behavioral results with S. discoideus support the concept that the compounds act as a sex pheromone blend rather than an aggregation pheromone blend, since the presence of males only elicited behavior in females. These results differ from previous interpretations that 4-methyl-3,5-heptanedione is an aggregation pheromone in Sitona species (Toth et al. 1998; Blight et al. 1984). Unfortunately, the sex of weevils trapped using the pheromone in Hungary was not reported (Toth et al. 1998), but Blight and colleagues (Blight et al. 1984) reported a 2:1 (male/female) sex ratio of catches to the male-produced compound 4-methyl-3,5-heptanedione and bean plant headspace volatiles. This could be explained by the hypothesis that sex pheromones are sometimes mistaken for aggregation pheromones, and the apparent aggregation is then caused by individuals of the same sex that are “eavesdropping” (Baker 1985). For aestivating or diapausing insects that annually migrate and establish in certain, often ephemeral plant groups that show patchy distribution (e.g., Medicago spp.), there is adaptive advantage in following odor cues of colonizing populations of conspecifics albeit of the same sex (Goldson, S.L. personal communication). Furthermore, as Landon et al. (1997) points out, there might be different behavioral responses to the same signal during different parts of the season.
Our results indicate the presence of ORN populations specialized in detecting 4-methyl-3,5-heptanedione in both male and female S. discoideus, which may imply the behavioral significance of this compound. Our results also showed the presence of separate ORN populations specialized for the four stereoisomers of 5-hydroxy-4-methyl-3-heptanone. At least some of these ORNs are stereoselective, being able to distinguish different stereoisomers. The presence of stereoselective ORNs in male and female S. discoideus suggests that the intraspecific communication system in S. discoideus is stereoselective. If any of these compounds act as a pheromone in this species, the presence of specialized ORNs for them and the similarity in EAG dose–responses in both sexes suggest that at least some of these compounds act as aggregation pheromones. It is also possible that two different combinations of these compounds are used as sex pheromones and aggregation pheromones in S. discoideus, respectively.
The presence of stereoselective ORNs also suggests that in an evolutionary context, it has been important for the weevils to differentiate between the four isomers of 5-hydroxy-4-methyl-3-heptanone, and some of the isomers may be used as a chemical signal to enhance discrimination between similar but different species. High sensitivity, in particular to one of the isomers (i.e., 4R,5R) that is not produced by the species, suggests a strong selection for this differentiating capacity. This may be revealed by further investigations of pheromones in related sympatric species in the future. We suggest this to be the most likely reason for the development of specific ORNs for each one of the isomers of 5-hydroxy-4-methyl-3-heptanone.
It is intriguing that (4S,5S)-5-hydroxy-4-methyl-3-heptanone (found as a minor component) is a stereoisomer of sitophilure, the pheromone of weevils in the genus Sitophilus in the same tribe (subfamily Brachyderinae, tribe Sitonini), which use (4R,5S)-5-hydroxy-4-methyl-3-heptanone as an aggregation pheromone (Walgenbach and Burkholder 1986; Mori and Ebata 1986; Mori et al. 1988; Walgenbach et al. 1987; Schmuff et al. 1984). We speculate that this could represent an earlier stage in evolution, with possible sympatric habitats in which they coexisted with the rice or maize weevil (Sitophilus spp.).
We conclude that the S. discoideus males produce the common Sitona pheromone component; 4-methyl-3,5-heptanedione. The minor component, which we have identified as the (4S,5S)-isomer of sitophilure, may be used to differentiate the signal towards S. discoideus conspecifics and to avoid cross-attraction to other Sitona species. These findings also provide the basis for future field studies to investigate whether 4-methyl-3,5-heptanedione, in combination with (4S,5S)-5-hydroxy-4-methyl-3-heptanone, provides an effective attractant. Ideally, this would be coordinated with a design that evaluates the effectiveness of different trap designs to attract and capture S. discoideus. Furthermore, the results from our study on the chemical communication system in this weevil species does not exclude that acoustic or visual signals between the weevils possibly are playing a key role in the attraction over short range.
References
Baker TC (1985) Chemical control of behaviour. In: Kerkut GA, Gilbert LS (eds) Comprehensive insect physiology, biochemistry and pharmacology. Pergamon Press, Ltd, Oxford, pp 621–672
Blight MM, Pickett JA et al (1984) An aggregation pheromone of Sitona lineatus: identification and initial field studies. Naturwissenschaften 71(9):480–480. doi:10.1007/bf00455905
Blight MM, Dawson GW, Pickett JA, Wadhams LJ (1991) The identification and biological activity of the aggregation pheromone of Sitona lineatus. Asp Appl Biol 27:137–142
Bohman B, Unelius CR (2009) Synthesis of all four stereoisomers of 5-hydroxy-4-methyl-3-heptanone using plants and oyster mushrooms. Tetrahedron 65(42):8697–8701. doi:10.1016/j.tet.2009.08.049
Chadwick CE (1960) Sitona humeralis Steph. (Coleoptera: Curculionidae) recorded from New South Wales. Aust J Sci 22:453–455
Esson MJ (1975) Notes on the biology and distribution of three recently discovered exotic weevil pests in Hawkes Bay. Proceedings of the 28th New Zealand Weed and Pest Control Conference. pp. 1208–1212
Frampton ER (1987) The reproductive seasonality and flight capability of Sitona discoideus Gyllenhal (Coleoptera: Curculionidae) and its pattern of larval establishment in Canterbury lucerne. PhD, University of Canterbury
Goldson SL, French RA (1983) Age-related susceptibility of lucerne to sitona weevil, Sitona discoideus Gyllenhal (Coleoptera: Curculionidae), larvae and the associated patterns of adult infestation. N Z J Agric Res 26(2):251–255
Goldson SL, Frampton ER et al (1984) The seasonal biology of Sitona discoideus Gyllenhal (Coleoptera: Curculionidae), an introduced pest of New Zealand lucerne. Bull Entomol Res 74(02):249–259. doi:10.1017/S000748530001138X
Goldson SL, Dyson CB et al (1985) The effect of Sitona discoideus Gyllenhal (Coleoptera: Curculionidae) on lucerne yields in New Zealand. Bull Entomol Res 75(03):429–442. doi:10.1017/S000748530001453X
Goldson SL, Proffitt JR et al (1990) Seasonal biology and ecology in New Zealand of Microctonus aethiopoides (Hymenoptera: Braconidae), a parasitoid of Sitona spp. (Coleoptera: Curculionidae), with special emphasis on atypical behavior. J Appl Ecol 27:703–722
Heathcock CH, Pirrung MC et al (1979) Acyclic stereoselection. 4. Assignment of stereostructure to β-hydroxycarbonyl compounds by carbon-13 nuclear magnetic resonance. J Org Chem 44(24):4294–4299
Kain WM, Trough TRE (1982) Insect pests of lucerne in New Zealand. In: Wynn-Williams RB (ed) Lucerne for the 80s. (Special Publication No.1). Agronomy Society of New Zealand Christchurch, New Zealand, pp 49–57
Kazlauskas RJ, Weissfloch ANE et al (1991) A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol esterase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J Org Chem 56(8):2656–2665. doi:10.1021/jo00008a016
Landon F, Ferary S et al (1997) Sitona lineatus host-plant odors and their components: effect on locomotor behavior and peripheral sensitivity variations. J Chem Ecol 23:2161–2173
Mori K, Ebata T (1986) Pheromone synthesis. 88. Synthesis of all of the four possible stereoisomers of 5-hydroxy-4-methyl-3-heptanone (sitophilure), the aggregation pheromone of the rice weevil and the maize weevil. Tetrahedron 42(16):4421–4426
Mori K, Yoshimura T et al (1988) Pheromone synthesis. CX. Synthesis of (4S,5R)-5-hydroxy-4-methyl-3-heptanone (sitophilure), the aggregation pheromone of Sitophilus oryzae and S. zeamais. Liebigs Ann Chem 9:899–902
Nielsen BS, Jensen TS (1993) Spring dispersal of Sitona lineatus: the use of aggregation pheromone traps for monitoring. Entomol Exp Appl 66(1):21–30. doi:10.1111/j.1570-7458.1993.tb00688.x
Schmuff NR, Phillips JK et al (1984) The chemical identification of the rice weevil and maize weevil aggregation pheromone. Tetrahedron Lett 25(15):1533–1534
Toth M, Smart LE, Szarukan I, Imrei Z (1998) Preliminary observations on species specificity of Sitona lineatus (L.) pheromone traps in Hungary (Coleoptera: Curculionidae). Acta Phytopathol Entomol Hung 33(3–4):349–356
Unelius CR, Sandell J et al (1998) Enantioselective preparation of the stereoisomers of 4-methylheptan-3-ol using Candida antarctica Lipase B. Collect Czechoslov Chem Commun 63(4):525–533
Walgenbach CA, Burkholder WE (1986) Factors affecting the response of the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae), to its aggregation pheromone. Environ Entomol 15(3):733–738
Walgenbach CA, Phillips JK et al (1987) Determination of chirality in 5-hydroxy-4-methyl-3-heptanone, the aggregation pheromone of Sitophilus oryzae (L.) and S. zeamais Motschulsky. J Chem Ecol 13(12):2159–2169
Acknowledgments
We thank B. J. Bunn for synthesis of 4-methyl-3,5-heptanedione and J. Daly and A. R. Gibb for their assistance in the initial studies. This research was funded by the Ministry of Building Innovation and Employment through the Bio-Protection Research Centre (LINX0304, contract 25949). The Linnaeus University, Kalmar, Sweden, is gratefully acknowledged for financial support of BB and CRU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Unelius, C.R., Park, KC., McNeill, M. et al. Identification and electrophysiological studies of (4S,5S)-5-hydroxy-4-methyl-3-heptanone and 4-methyl-3,5-heptanedione in male lucerne weevils. Naturwissenschaften 100, 135–143 (2013). https://doi.org/10.1007/s00114-012-1003-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-012-1003-4