Abstract
Aims/hypothesis
Obesity is characterised by lipid accumulation in skeletal muscle, which increases the risk of developing insulin resistance and type 2 diabetes. AMP-activated protein kinase (AMPK) is a sensor of cellular energy status and is activated in skeletal muscle by exercise, hormones (leptin, adiponectin, IL-6) and pharmacological agents (5-amino-4-imidazolecarboxamide ribonucleoside [AICAR] and metformin). Phosphorylation of acetyl-CoA carboxylase 2 (ACC2) at S221 (S212 in mice) by AMPK reduces ACC activity and malonyl-CoA content but the importance of the AMPK–ACC2–malonyl-CoA pathway in controlling fatty acid metabolism and insulin sensitivity is not understood; therefore, we characterised Acc2 S212A knock-in (ACC2 KI) mice.
Methods
Whole-body and skeletal muscle fatty acid oxidation and insulin sensitivity were assessed in ACC2 KI mice and wild-type littermates.
Results
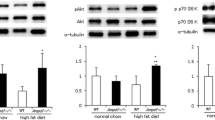
ACC2 KI mice were resistant to increases in skeletal muscle fatty acid oxidation elicited by AICAR. These mice had normal adiposity and liver lipids but elevated contents of triacylglycerol and ceramide in skeletal muscle, which were associated with hyperinsulinaemia, glucose intolerance and skeletal muscle insulin resistance.
Conclusions/interpretation
These findings indicate that the phosphorylation of ACC2 S212 is required for the maintenance of skeletal muscle lipid and glucose homeostasis.







Similar content being viewed by others
Abbreviations
- 2DG:
-
2-Deoxyglucose
- ACC:
-
Acetyl-CoA carboxylase
- ACC DKI:
-
Acc double knock-in mice
- ACC2 KI:
-
Acc2 S212A KI
- AMPK β1β2 M-KO:
-
Skeletal muscle-specific Ampk β1β2 KO
- AICAR:
-
5-Amino-4-imidazolecarboxamide ribonucleoside
- AMPK:
-
AMP-activated protein kinase
- CPT-I:
-
Carnitine palmitoyl transferase
- Cyt:
-
Cytochrome
- DKI:
-
Double KI
- EDL:
-
Extensor digitorum longus
- GDR:
-
Glucose disposal rate
- HFD:
-
High-fat diet
- KI:
-
Knock-in
- KO:
-
Knockout
- OXPHOS:
-
Proteins involved in oxidative phosphorylation
- RQ:
-
Respiratory quotient
- TAG:
-
Triacylglycerol
- TBC1D 1 and 4:
-
tre-2/USP6, BUB2, cdc16 domain family member 1 and 4
- \( \overset{\cdot }{V}{\mathrm{CO}}_2 \) :
-
Rate of CO2 production
- \( \overset{\cdot }{V}{\mathrm{O}}_2 \) :
-
Rate of O2 consumption
- WT:
-
Wild-type
References
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89:1025–1078
Steinberg GR, Jorgensen SB (2007) The AMP-activated protein kinase: role in regulation of skeletal muscle metabolism and insulin sensitivity. Mini-Rev Med Chem 7:519–526
Abu-Elheiga L, Jayakumar A, Baldini A, Chirala SS, Wakil SJ (1995) Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. Proc Natl Acad Sci U S A 92:4011–4015
Iverson AJ, Bianchi A, Nordlund AC, Witters LA (1990) Immunological analysis of acetyl-CoA carboxylase mass, tissue distribution and subunit composition. Biochem J 269:365–371
Abu-Elheiga L, Brinkley WR, Zhong L, Chirala SS, Woldegiorgis G, Wakil SJ (2000) The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci U S A 97:1444–1449
Choi CS, Savage DB, Abu-Elheiga L et al (2007) Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A 104:16480–16485
Abu-Elheiga L, Oh W, Kordari P, Wakil SJ (2003) Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A 100:10207–10212
Hoehn KL, Turner N, Swarbrick MM et al (2010) Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab 11:70–76
Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB (2010) Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc Natl Acad Sci U S A 107:7598–7603
Dzamko N, Schertzer JD, Ryall JG et al (2008) AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol 586:5819–5831
Jeppesen J, Maarbjerg SJ, Jordy AB et al (2013) LKB1 regulates lipid oxidation during exercise independently of AMPK. Diabetes
O'Neill HM, Maarbjerg SJ, Crane JD et al (2011) AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A 108:16092–16097
Steinberg GR, O'Neill HM, Dzamko NL et al (2010) Whole-body deletion of AMPK β2 reduces muscle AMPK and exercise capacity. J Biol Chem 285:37198–37209
Lee CW, Wong LL, Tse EY et al (2012) AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res 72:4394–4404
Fullerton MD, Galic S, Marcinko K et al (2013) Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19:1649–1654
Hawley SA, Fullerton MD, Ross FA et al (2012) The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336:918–922
Galic S, Fullerton MD, Schertzer JD et al (2011) Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest 121:4903–4915
Palanivel R, Fullerton MD, Galic S et al (2012) Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance. Diabetologia 55:3083–3093
Passonneau JV, Gatfield PD, Schulz DW, Lowry OH (1967) An enzymic method for measurement of glycogen. Anal Biochem 19:315–326
Watt MJ, Dzamko N, Thomas WG et al (2006) CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 12:541–548
Anderson EJ, Lustig ME, Boyle KE et al (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119:573–581
Smith BK, Jain SS, Rimbaud S et al (2011) FAT/CD36 is located on the outer mitochondrial membrane, upstream of long-chain acyl-CoA synthetase, and regulates palmitate oxidation. Biochem J 437:125–134
Bouzakri K, Austin R, Rune A et al (2008) Malonyl CoenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes 57:1508–1516
Joly E, Bendayan M, Roduit R, Saha AK, Ruderman NB, Prentki M (2005) Malonyl-CoA decarboxylase is present in the cytosolic, mitochondrial and peroxisomal compartments of rat hepatocytes. FEBS Lett 579:6581–6586
Park H, Kaushik VK, Constant S et al (2002) Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem 277:32571–32577
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387
Kerner J, Distler AM, Minkler P, Parland W, Peterman SM, Hoppel CL (2004) Phosphorylation of rat liver mitochondrial carnitine palmitoyltransferase-I: effect on the kinetic properties of the enzyme. J Biol Chem 279:41104–41113
Bezaire V, Heigenhauser GJ, Spriet LL (2004) Regulation of CPT I activity in intermyofibrillar and subsarcolemmal mitochondria from human and rat skeletal muscle. Am J Physiol Endocrinol Metab 286:E85–E91
Holloway GP, Bezaire V, Heigenhauser GJ et al (2006) Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol 571:201–210
Holland WL, Brozinick JT, Wang LP et al (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5:167–179
Hoehn KL, Hohnen-Behrens C, Cederberg A et al (2008) IRS1-independent defects define major nodes of insulin resistance. Cell Metab 7:421–433
JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A (2007) Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 56:394–403
Davies SP, Sim AT, Hardie DG (1990) Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem 187:183–190
Munday MR, Campbell DG, Carling D, Hardie DG (1988) Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem 175:331–338
Ha J, Daniel S, Broyles SS, Kim KH (1994) Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem 269:22162–22168
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565
Thomson DM, Brown JD, Fillmore N et al (2007) LKB1 and the regulation of malonyl-CoA and fatty acid oxidation in muscle. Am J Physiol Endocrinol Metab 293:E1572–E1579
Kaushik VK, Young ME, Dean DJ, Kurowski TG, Saha AK, Ruderman NB (2001) Regulation of fatty acid oxidation and glucose metabolism in rat soleus muscle: effects of AICAR. Am J Physiol Endocrinol Metab 281:E335–E340
Merrill GF, Kurth EJ, Hardie DG, Winder WW (1997) AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 273:E1107–E1112
Dzamko NL, Steinberg GR (2009) AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta Physiol (Oxf) 196:115–127
Schenk S, Horowitz JF (2007) Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117:1690–1698
Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH (2007) Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117:1679–1689
Ussher JR, Koves TR, Cadete VJ et al (2010) Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59:2453–2464
Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ (2007) Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem 282:12583–12589
Summers SA (2006) Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45:42–72
Steinberg GR, Michell BJ, van Denderen BJ et al (2006) Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4:465–474
Sugden MC, Howard RM, Munday MR, Holness MJ (1993) Mechanisms involved in the coordinate regulation of strategic enzymes of glucose metabolism. Adv Enzym Regul 33:71–95
Holness MJ, Kraus A, Harris RA, Sugden MC (2000) Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes 49:775–781
Jeoung NH, Harris RA (2008) Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab 295:E46–E54
Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM (2006) Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55:2277–2285
Funding
These studies were supported by grants and fellowships from the Australian Research Council and CSIRO (BEK), National Health and Medical Research Council (BEK, GRS, BJvD), the Canadian Diabetes Association (JRBD, GRS) and the Canadian Institutes of Health Research (CIHR) (JRBD, GRS). Support in part was received from the Victorian Government’s OIS Program (BEK) and Canadian Foundation for Innovation (GRS). MDF is a CIHR Banting Postdoctoral Fellow and GRS holds a Canada Research Chair in Metabolism and Obesity.
Contribution statement
HMO and GRS designed experiments and wrote manuscript. GRS, BEK and SBJ provided funding for the project and revised the manuscript. HMO performed the majority of experiments. JSL, SG, MT, PDA, MDF, BKS, TP, ZC, BJvD, MCS, SBJ, JRBD, GPH and TJH designed and performed experiments and revised the manuscript. All authors approved the final version of the manuscript. GRS is responsible for the integrity of the work as a whole.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 136 kb)
ESM Fig. 1
(PDF 306 kb)
ESM Table 1
(PDF 90 kb)
ESM Table 2
(PDF 196 kb)
ESM Table 3
(PDF 146 kb)
Rights and permissions
About this article
Cite this article
O’Neill, H.M., Lally, J.S., Galic, S. et al. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia 57, 1693–1702 (2014). https://doi.org/10.1007/s00125-014-3273-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3273-1