Abstract
Aims/hypothesis
Shortened telomere length is a marker of cell damage and is associated with oxidative stress, chronic inflammation and metabolic disease. We hypothesised that the offspring of women with gestational diabetes mellitus (GDM) with increased risk of cardiovascular and metabolic diseases might exhibit shorter telomere length.
Methods
We investigated telomere length in 439 GDM and 469 control group offspring, aged between 9 and 16 years, recruited from the Danish National Birth Cohort. Relative telomere length was measured in peripheral blood DNA (n = 908) using a quantitative PCR approach. Multivariate regression analysis was used to investigate the association between mothers’ GDM status and telomere length in the offspring.
Results
Female offspring had longer telomeres than males. Offspring of mothers with GDM had significantly shorter telomere length than control offspring, but this difference was observed only in girls. There was a negative association between telomere length and GDM exposure among the female offspring (14% shorter telomeres, p = 0.003) following adjustment for the age of the offspring. Telomere length in female offspring was negatively associated with fasting insulin levels and HOMA-IR (p = 0.03). Maternal age, smoking, gestational age, birthweight and the offspring’s anthropometric characteristics were not associated with telomere length (p ≥ 0.1).
Conclusions/interpretation
The 9- to 16-year-old girls of mothers with GDM had shorter telomeres than those from the control population. Further studies are needed to understand the extent to which shortened telomere length predicts and/or contributes to the increased risk of disease later in life among the offspring of women with GDM.
Similar content being viewed by others

Introduction
Gestational diabetes mellitus (GDM) complicates 1–10% of all pregnancies [1, 2] and is increasing in prevalence worldwide, most likely due to obesity, inactivity, urbanisation and pregnancy occurring at an older age [3, 4]. The offspring of mothers with GDM are at increased risk of developing metabolic diseases, and a growing number of studies implicate the time in utero as playing a key role in this process [5,6,7,8,9,10]. However, the underlying molecular mechanisms are still unknown.
Telomeres are formed of non-coding tandem repeats of the hexamer TTAGGG located on the chromosome ends, with a total length from a few kb to 15 kb. Telomeres function to protect the integrity of the genome by forming a loop that prevents end-fusions of the chromosomes [11]. Telomeres shorten with each cell division since DNA replication is unable to fully replicate to the chromosome ends [12]. In response to DNA and telomere damage, the ribonucleoprotein enzyme telomerase may be upregulated to preserve telomere length by adding TTAGGG repeats, but only in some cell types [13]. The telomere length of blood cells is strongly associated with age due to the close association with the number of cell divisions [14], but an unhealthy lifestyle may also influence the rate of telomere attrition. Previous studies have shown that age- and lifestyle-related diseases such as type 2 diabetes [15], cardiovascular disease [16, 17], atherosclerosis [18], obesity-derived inflammation and oxidative stress [19] are associated with telomere shortening. It is therefore intuitive that shortened telomeres are associated with an increased risk of all-cause mortality [20]. It is debated whether the telomere shortening may be a cause or a consequence of metabolic disease, but telomere length has recently been shown to predict the onset of type 2 diabetes in high-risk populations [15].
Telomere length is largely determined in stem cell populations early in fetal development [21] and is highly variable between individuals at birth [22]. Interestingly, female individuals have a longer average telomere length at all ages [23]. There is evidence to suggest that telomere length is influenced in utero by environmental and maternal factors such as intrauterine growth restriction [24], smoking [25], maternal stress [26] and maternal education [27]. As each of these is a recognised risk factor for a range of adverse offspring health outcomes, it is plausible that telomere shortening may be a mediator in fetal programming of later health. Interestingly, pregnancies in mothers with GDM have been associated with shorter telomere length in cord blood [28], and pregnancies in mothers with type 2 diabetes have been associated with shorter telomere length in placenta tissue [29]. Additionally, pregnancies in mothers with GDM and with type 1 diabetes have been associated with increased telomerase activity in cord blood [30]. Other studies have, however, reported no association between GDM and cord blood telomere length [31] or between maternal type 1 diabetes and telomere length in young adult offspring [32]. Importantly, these studies were mainly of small sample size and, to the best of our knowledge, the effect of GDM on telomere length in adolescent offspring has not previously been reported.
Large-scale longitudinal birth cohort studies are needed to address how maternal GDM can potentially explain variability in offspring telomere length, taking into account the effect of the multiple environmental exposures that have undoubtedly occurred after birth. In the present study, we examined the association between in utero GDM exposure and telomere length in 9- to 16-year-old offspring, and assessed the relationship between telomere length and childhood metabolic health.
Methods
Study cohort
Between January 1996 and October 2002, 91,827 women were enrolled in the Danish National Birth Cohort (DNBC). A GDM subgroup (1350 pregnancies) as part of the Diabetes & Women’s Health Study [33], together with a randomly selected control group (2629 pregnancies), was established from the DNBC to study the possible roles of maternal and offspring lifestyle associated with metabolic health in both generations. Details of the identification of suspected GDM in the DNBC have previously been described [34]. Briefly, women with GDM were defined by a GDM-related diagnosis recorded in the Danish National Patient Registry in relation to the index pregnancy (ICD-10 codes O24.4 and O24.9; www.who.int/classifications/icd/en/) or a self-reported GDM event during study interviews at 30 weeks of gestation or 6 months postpartum, or both. All eligible GDM mother-child dyads in the DNBC were invited; in total 1289. A total of 1457 control dyads were invited, to match the number of recruited GDM dyads.
Between March 2012 and May 2014, a total of 608 (47%) children of mothers with GDM and 626 (43%) children of mothers from the control group not affected by GDM were recruited for phenotypic and molecular studies. The main reason for non-participation was lack of time. The majority of the participants were examined at one of the two main hospitals in Denmark: Copenhagen University Hospital or Aarhus University Hospital. For women who lived at a greater distance from the primary hospitals, the clinical examination was done at smaller pop-up clinics located closer to their home. There were seven pop-up clinics, representing all major regions in Denmark. The participants were invited in a 1:3 ratio, i.e. one control dyad and three GDM dyads, throughout the country, then followed by the majority of control dyads examined at Copenhagen University Hospital. All technical staff were centrally trained by staff from the two main hospitals, and staff worked at multiple sites in order to ensure uniformity and high quality of data collection. Clinical examinations and biospecimen collection was conducted following standardised protocols.
The study design and protocol were approved by the Regional Scientific Ethical Committee of the municipalities of Copenhagen and Frederiksberg (H-4-2011-045 and H-4-2013-129) and conformed to the Helsinki II declaration. Consent from both parents was essential for the child’s participation in the study.
Clinical examinations
The offspring of women with GDM and control participants underwent a clinical examination including anthropometric measurements, BP measurements and fasting blood samples for measurement of glucose, high-sensitivity C-reactive protein (hs-CRP), insulin and C-peptide levels and lipid profiles. Standard assays were used for biomarker analysis as previously described [35]. The HOMA-IR index was calculated as: [(fasting plasma insulin [pmol/l] × fasting plasma glucose [mmol/l]) /22.5] × 0.144.
In order to determine the puberty status of the study participants, clinical pubertal assessment was made using Tanner’s staging [36]. Among the female offspring, breast development ≥B2 or pubic hair stage ≥PH2 was considered to be a marker of pubertal onset. In male offspring, testis volume ≥ 4 ml, pubic hair stage ≥PH2 or boys genital stage ≥G2 was considered to be a marker of pubertal onset.
Study participants examined at the Copenhagen University Hospital (n = 666) were also studied for body composition using dual-energy x-ray absorptiometry scanning (Lunar; Scanex, Hørsholm, Denmark).
Sample collection and DNA isolation
A standardised blood sample collection procedure was used across all study examination sites. Peripheral venous blood was collected into EDTA tubes (10 ml; BD, Franklin Lakes, NJ, USA) that were placed directly on ice and then centrifuged within 10 min of collection. Buffy coats were collected from EDTA tubes and stored immediately at −20°C with subsequent transfer (within 8 h) to −80°C until later DNA isolation. Buffy coats were collected from 536 offspring of mothers with GDM and 555 control offspring, and DNA was extracted from all samples using the QIAamp 96 DNA Blood Kit in line with the manufacturer’s protocol (Qiagen, Valencia, CA, USA).
Telomere measurements by quantitative PCR
We evaluated DNA quality and concentration using the Qubit dsDNA BR Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Average relative telomere length was measured by a quantitative real-time PCR protocol modified from the previously described method by Cawthon [37]. In brief, this method generates a measure of the average telomere length as a ratio (T/S) of telomere repeat length (T) to copy number of a single-copy gene (S), within each DNA sample. We used β-globin (BG; also known as HBB) as the reference single-copy gene. Primers for the telomere repeat and BG are shown in electronic supplementary material [ESM] Table 1. All measurements were performed in quadruplicates in 384-well plate formats, using the LightCycler 480 Real-Time PCR System (Roche, Melbourne, VIC, Australia). DNA 5 ng amounts were used in each reaction with amplification using SensiMix SYBR Fluorescein Mastermix reagents (Bioline, Sydney, NSW, Australia). Reactions were set up on ice to prevent premature DNA polymerase activation, non-specific amplification and primer-dimerisation. Samples were randomised across plates by exposure group and sex. Two sets of standard oligomers (the telomere TTAGGG repeat and the reference gene BG; ESM Table 1) were run as a seven-point standard curve on each plate to ensure that sample DNA Ct values were within the linear range of the two standard curves, as well as to assess variation between plate runs. Additionally, two inter-run controls were measured on all plates (K562 genomic DNA sample) to account for inter-run variability. A negative (no template) control was included on all plates. Relative average telomere lengths were calculated using the ΔCt method.
After evaluating DNA quality and quantitative PCR quadruplicate performances, we included 908 offspring telomere measurements in the final analyses (n = 439 GDM and 469 control offspring). We achieved CVs within quadruplicates of the telomere runs of 6% and of single-copy gene runs of 4%. We achieved inter-plate CVs of the telomere runs of 5% and of single-copy gene runs of 2%). A sensitivity analysis was conducted in which each plate-run was separately excluded to test for any batch effect by individual plate on the total telomere length results. None of the individual plates affected the final results.
Statistical analysis
Normally distributed data are presented as means ± SD and were compared by Student’s t test. Non-normally distributed data are presented as medians and interquartile ranges and were compared by Mann–Whitney U test. Proportions of categorical data were calculated by χ2 test.
Multivariate linear regression analyses were applied to address the association between GDM and telomere lengths in the offspring with adjustment for the offspring’s sex and age. We tested potential confounding effects of pre-pregnancy maternal BMI (mBMI), maternal age, birthweight, gestational age and maternal smoking status (all previously reported to impact telomere length) as well as Caesarean section, maternal socio-occupational status, weight gain in pregnancy, paternal pre-pregnancy BMI and breastfeeding. We examined whether pubertal onset was associated with telomere length in separate univariate regression analyses for males and females. Furthermore, we investigated whether offspring phenotype was associated with telomere length by conducting multivariate regression analyses for a range of offspring characteristics, adjusting for offspring sex and age. In all regression models, we estimated mean differences as β-coefficients and 95% CI. In all models, we tested and confirmed that exclusion of siblings did not affect the results; therefore we included all siblings in the analyses. Assumptions of equal variance and normally distributed residuals were visualised in QQ plots and histograms. All statistical analyses were performed using SAS 9.3 statistical software (SAS Institute, Cary, NC, USA), and p ≤ 0.05 was considered statistically significant.
Results
Maternal, birth and offspring characteristics of the DNBC GDM subcohort
Mothers with GDM had a higher pre-pregnancy mBMI, were older and tended to be more likely smokers and of lower socio-occupational status than control mothers. Furthermore, mothers with GDM underwent a higher proportion of Caesarean section deliveries, and had a lower gestational age and higher birthweight of their offspring, compared with the control group (Table 1). At the age of 9–16 years, the offspring of GDM mothers had a range of adverse metabolic changes independent of sex and age; these included increased fasting plasma glucose and insulin levels, HOMA-IR, BMI and total body fat percentage (BF%) (Table 1). These results are consistent with the previously described characteristics for the entire cohort of GDM and control offspring [35]. In this previous baseline study, we also found that, when adjusting for age and sex, all anthropometric and metabolic differences between the two offspring groups remained, except that an earlier onset of puberty in the female offspring of mothers with GDM became significant [35]. Therefore we did not separate the results in Table 1 into female and male offspring.
Telomere length is associated with intrauterine exposure to GDM in female offspring
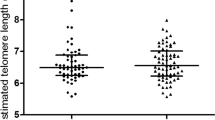
As anticipated, female offspring had significantly longer telomeres than male offspring (p = 0.0007, unadjusted) (Fig. 1a). Offspring in the GDM group had significantly shorter telomeres than control offspring (p = 0.03, unadjusted) (Fig. 1b). Further analysis showed that this relationship was primarily driven by shortened telomeres in female offspring of mothers with GDM relative to control female offspring, such that telomere length in GDM female offspring was equivalent to that in male GDM and male controls (Fig. 1c).
Because of the observed sex differences, we examined whether telomere length differed in association with progression into puberty. No association with onset of puberty was found in either female or male offspring (female: p = 0.94, β 8.3, 95% CI −197, 214; male: p = 0.65, β −65, 95% CI −339, 212).
Next we investigated the association between telomere length in the offspring and the following exposures and/or potential confounders: GDM status, pre-pregnancy mBMI, maternal age, gestational age, birthweight, smoking, Caesarean section, maternal socio-occupational status, weight gain in pregnancy, paternal BMI or breastfeeding, with adjustment for sex and age of the offspring. Only GDM status (β −154 [−7.7%], 95% CI −283, −25, p = 0.02), pre-pregnancy mBMI (β −12 [−0.6%], 95% CI −25, 0.8, p = 0.07) and maternal weight gain in pregnancy (β 8.9 [0.4%], 95% CI −0.6, 19, p = 0.07) showed a significant or borderline significant association with telomere length after adjustment for sex and age of the offspring (Table 2).
Previously reported confounders of offspring telomere length do not affect the association between GDM status and offspring telomere length
To further address the impact of GDM and pre-pregnancy mBMI respectively on telomere length in the offspring, we applied multivariate regression models with adjustment for GDM status, pre-pregnancy mBMI, sex, offspring age as well as maternal age, gestational age, birthweight and smoking as these factors are the most well known from the literature to impact telomere length. Although weight gain in pregnancy also showed a borderline significant association with telomere length in univariate analyses, we excluded this variable in the models since weight gain is closely and inversely associated with pre-pregnancy mBMI. Including both pre-pregnancy mBMI and GDM status as covariates, both these variables became non-significantly associated with telomere length, most likely given their well-established relatedness (Table 3, model A). We further assessed the impact of GDM and pre-pregnancy mBMI on offspring telomere length separately to avoid overadjustment. Telomere length in the offspring remained significantly associated with GDM (β −151, 95% CI −287, −15, p = 0.03; Table 3, model B), and also showed a borderline significant association with pre-pregnancy mBMI (β −12, 95% CI −25, 1.4, p = 0.08; Table 3, model C), after adjustment for offspring sex and age, as well as the previously published confounders: maternal age at birth, gestational age, birthweight and smoking. In all models, the strongest contributor to telomere length was sex (Table 3).
Given the significantly larger telomere length difference in female offspring between GDM and control offspring, and the lack of difference between the two male groups, we also conducted the analyses in Table 3 separately for each sex (ESM Table 2). The association between telomere length and GDM status became more significant in female offspring (β −306 [−14.0%], 95% CI −508, −104, p = 0.003), and disappeared among the male offspring (β: −4.5 [−0.2%], 95% CI −189, 180, p = 0.96).
Association between telomere length and phenotype of the offspring
To explore the potential link between telomere length and phenotypic measures in the offspring, we performed multivariate regression analyses separately for each sex between telomere length and offspring total BF%, systolic BP, fasting plasma insulin, glucose or hs-CRP levels, with adjustment for offspring age. For the female offspring, significant negative associations between telomere length and insulin levels as well as HOMA-IR were observed. No significant associations were observed for the male offspring between telomere length and any of these variables (Table 4).
Discussion
We showed that female offspring of women with GDM have a shorter telomere length at the age of 9–16 years. This association was not influenced by age, maternal age at birth, gestational age, birthweight or smoking in pregnancy. Telomere length in female offspring only was negatively associated with fasting insulin levels and HOMA-IR.
Important strengths of our study are the large sample size and that this is, to our knowledge, the first to explore telomeres in adolescent children of GDM mothers. However, our findings in adolescents support the results of several previous findings in smaller studies. For example, Xu et al showed a shorter telomere length in cord blood leucocytes in GDM compared with control offspring, with a similar magnitude (approximately 5–10% shorter) to our observations (n = 82 GDM and n = 65 control offspring) [28]. Additionally, our results are in line with those of Gilfillan et al [38], who reported a negative correlation between cord blood telomere length and maternal glucose levels in a population of women with pre-gestational diabetes, a group with gestational diabetes and a control group, although they were not able to detect absolute differences in the telomere lengths between the groups (n = 52). Cross et al observed increased telomerase activity in cord blood from pregnancies in women with GDM and with type 1 diabetes, potentially reflecting a compensation for in utero telomere shortening and DNA damage, but did not observe any relationship between maternal diabetes (type 1 or 2 diabetes or GDM) and fetal telomere length [30]. In contrast, Cross et al also reported a lack of difference in telomere length and DNA damage in a smaller study of young adult offspring of women with type 1 diabetes in pregnancy compared with control participants [32]. The inconsistent results may be explained by a lack of power and the age of the offspring. Furthermore, these earlier studies did not report sex-specific differences in telomere length, as we found in our study.
Maternal factors, offspring phenotype and telomere length in childhood
Previous studies have demonstrated effects of smoking [25], maternal socio-occupational status [27] and maternal age [39] at time of pregnancy, as well as gestational age [40] and low birthweight [24], on telomere length at time of birth, but we did not find any associations between telomere length in childhood and these factors. However, the variations in maternal age, gestational age and birthweight were also narrow (SD 4.3 years, 12 days and 550 g, respectively) in our study. Additionally, we had no information on the current socio-occupational status of the children at the age of 9–16 years, and can therefore not exclude the fact that present socio-occupational status could confound the results. The number of mothers smoking during pregnancy was higher in our study (238 women smoking occasionally or daily) compared with previous publications (n ≤ 138), and cannot therefore explain the lack of replication of earlier findings. To this extent, our results are unique as they include the numerous unknown environmental exposures encountered through childhood and adolescence that may have affected telomere length. As telomere length is most variable at the time of birth, it may be speculated that telomeres at this time point are also more vulnerable to being affected by confounding factors, whereas telomere length is less variable in adolescence.
Maternal obesity is highly associated with GDM, and our results, which show an association between pre-pregnancy mBMI and shorter telomeres, further confirm that the two maternal factors are also highly related in terms of their effects on outcome in offspring. A recent large-scale study (n = 743) showed that pre-pregnancy mBMI was associated with shorter telomere length in cord blood (0.5% shorter telomeres per each kg/m2 increase in pre-pregnancy mBMI; 95% CI −0.83%, −0.17%) [41]. Our data confirm this magnitude of difference, showing a 0.6% decrease in telomere length per kg/m2 increase, but most importantly we show here that the effect of pre-pregnancy mBMI persists through childhood at least until adolescence among female offspring. However, our results point towards the fact that GDM status has a stronger impact on female offspring telomere length (14% shorter telomeres) than pre-pregnancy mBMI, and therefore biologically it is more likely that hyperglycaemia or other GDM-related factors and not pre-pregnancy mBMI may be affecting the telomere shortening.
In the present study, we found negative associations between telomere length and fasting insulin levels and HOMA-IR in female offspring. However, we found no significant associations with adiposity or hs-CRP as an inflammatory marker. Our results are in line with the study by Cross et al, who found no relationship between telomere length and inflammatory markers in healthy young adult offspring of type 1 diabetic mothers [32]. However, others have shown that, after weight loss compared with before, obese adolescents exhibit longer telomeres, and that this was associated with an improvement in glucose tolerance and decreased inflammation [42]. Our observation of negative associations between telomere length and offspring insulin levels and HOMA-IR raises the possibility that short telomere length may be a risk factor for or even predict the development of type 2 diabetes and associated metabolic traits and disease among the female offspring of women with GDM, independent of their body composition. However, this needs to be confirmed in other studies.
Potential mechanisms behind the telomere shortening
Pregnancies in women with GDM are associated with increased levels of oxidative stress and inflammation, owing to overproduction of free radicals and reactive oxygen species and/or to defects in antioxidant defences [43]. Oxidative stress is believed to be a major cause of telomere shortening [19], and higher levels of oxidative stress and inflammation in utero have been shown to induce changes in metabolism among the offspring [44]. Telomeres contain G-rich segments that are highly sensitive to reactive oxygen species and DNA breakage, and the increased oxidative stress involved in GDM is a potential molecular mechanism contributing to telomere shortening during fetal development, perhaps under compensation by induced telomerase activity as previously suggested [30]. Unfortunately, we were not able to quantify telomerase activity at the time of birth or at follow-up in the present study.
Other mechanisms not investigated here may also be important in regulating telomere length in the offspring of women with GDM. These could include epigenetics, which is indeed important in regulating telomere length through DNA methylation of CpG islands located within the telomeres [45]. Additionally, genetic variability in telomere length most likely plays a role in segregation with GDM traits [39]. We acknowledge that the shorter telomeres in females may not be due to programming mechanisms per se, but could equally likely be a consequence of a different lifestyle among the female offspring. We cannot exclude the possibility that environmental factors not measured in this study, i.e. physical activity, could be involved in the telomere shortening in the female offspring. Family factors including social class and eating behaviour may also play essential roles in the telomere shortening in female GDM offspring.
Sexual dimorphism in telomere length in response to GDM
Sex differences in telomere length are well appreciated, with female individuals possessing longer telomeres than male individuals from birth, and the differences persisting into adult life [23]. This phenomenon is believed to be related to the effect of oestrogen on upregulating the telomerase reverse transcriptase (TERT) gene and thereby telomerase activity [46]. As a quality control, we also replicated the known relationship of longer telomere length in female compared with male offspring in our study. Additionally, only the female group presented shortened telomere length among the GDM offspring, and these sex differences were not affected by progression into puberty. To our knowledge, this study is the first to report a sex-specific effect of telomere length in pregnancies in women with hyperglycaemia.
Studies have shown that female babies have higher cord blood C-peptide concentrations [47, 48]. Interestingly, girls exposed to maternal GDM or hyperglycaemia in utero are at higher risk of childhood adiposity, with increased risk if the mother is overweight or obese [49]. Landon et al reported that treatment of maternal hyperglycaemia had a long-term impact on glucose metabolism only in pre-pubertal female offspring, who had lower fasting glucose and HOMA-IR, suggesting a sex-specific impact [50]. It may be speculated that the shortened telomeres in the female offspring of mothers with GDM observed in our study is related to sex-specific differences in metabolic phenotype that may arise later in life. It would be interesting to explore telomere length at time of birth in this cohort, in order to rule out an increasing rate of postnatal telomere attrition in the female offspring, as well as in adult offspring, of mothers with GDM to assess the relationship between telomere length and risk of metabolic and other diseases later in life among these children.
Conclusions
Female offspring of women with GDM have shorter telomere length in childhood relative to control participants independent of other maternal factors. Telomere length in female offspring was also negatively associated with fasting insulin levels and HOMA-IR. This suggests that GDM and GDM-associated factors have sex-specific, long-term effects on offspring telomere length. Further studies are required to identify the mechanisms through which this arises and to assess the possible association with later onset of metabolic diseases such as type 2 diabetes.
Data availability
The datasets obtained and analysed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- BF%:
-
Body fat percentage
- DNBC:
-
Danish National Birth Cohort
- GDM:
-
Gestational diabetes mellitus
- hs-CRP:
-
High-sensitivity C-reactive protein
- mBMI:
-
Maternal BMI
References
Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC (2008) Gestational diabetes in the United States: temporal trends 1989 through 2004. Am J Obstet Gynecol 198:525.e1–525.e5
American Diabetes Association (2003) Gestational diabetes mellitus. Diabetes Care 26(Suppl 1):S103–S105
Ben-Haroush A, Yogev Y, Hod M (2004) Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med 21:103–113
Buchanan TA, Xiang AH (2005) Gestational diabetes mellitus. J Clin Invest 115:485–491
Clausen TD, Mathiesen ER, Hansen T et al (2008) High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31:340–346
Dabelea D, Hanson RL, Lindsay RS et al (2000) Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49:2208–2211
Sobngwi E, Boudou P, Mauvais-Jarvis F et al (2003) Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet (London, England) 361:1861–1865
Lawlor DA, Fraser A, Lindsay RS et al (2010) Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 53:89–97
Boerschmann H, Pflüger M, Henneberger L, Ziegler A-G, Hummel S (2010) Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care 33:1845–1849
Patel S, Fraser A, Davey Smith G et al (2012) Associations of gestational diabetes, existing diabetes, and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes Care 35:63–71
Blackburn EH (1991) Structure and function of telomeres. Nature 350:569–573
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56
Shore D, Bianchi A (2009) Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J 28:2309–2322
Frenck RW, Blackburn EH, Shannon KM (1998) The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A 95:5607–5610
Willeit P, Raschenberger J, Heydon EE et al (2014) Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PLoS One 9:e112483
Brouilette SW, Moore JS, McMahon AD et al (2007) Telomere length, risk of coronary heart disease, and statin treatment in the west of Scotland primary prevention study: a nested case-control study. Lancet (London, England) 369:107–114
Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349:g4227
Chen S, Lin J, Matsuguchi T et al (2014) Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: the strong heart family study. Aging (Albany NY) 6:414–427
Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP, Perrea DN (2012) “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res Rev 11:220–229
Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Diotti R, Loayza D. Shelterin complex and associated factors at human telomeres. Nucleus. 2:119–135
Okuda K, Bardeguez A, Gardner JP et al (2002) Telomere length in the newborn. Pediatr Res 52:377–381
Elbers CC, Garcia ME, Kimura M et al (2014) Comparison between southern blots and qPCR analysis of leukocyte telomere length in the health ABC study. J Gerontol A Biol Sci Med Sci 69:527–531
Biron-Shental T, Sukenik-Halevy R, Sharon Y, Laish I, Fejgin MD, Amiel A (2014) Telomere shortening in intra uterine growth restriction placentas. Early Hum Dev 90:465–469
Salihu HM, Pradhan A, King L et al (2015) Impact of intrauterine tobacco exposure on fetal telomere length. Am J Obstet Gynecol 212:205.e1–205.e8
Entringer S, Epel ES, Lin J et al (2013) Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol 208:134.e1–134.e7
Wojcicki JM, Olveda R, Heyman MB et al (2016) Cord blood telomere length in Latino infants: relation with maternal education and infant sex. J Perinatol 36:235–241
Xu J, Ye J, Wu Y et al (2014) Reduced fetal telomere length in gestational diabetes. PLoS One 9:e86161
Biron-Shental T, Sukenik-Halevy R, Naboani H, Liberman M, Kats R, Amiel A (2015) Telomeres are shorter in placentas from pregnancies with uncontrolled diabetes. Placenta 36:199–203
Cross JA, Temple RC, Hughes JC et al (2010) Cord blood telomere length, telomerase activity and inflammatory markers in pregnancies in women with diabetes or gestational diabetes. Diabet Med 27:1264–1270
Harville EW, Williams MA, Qiu C-F, Mejia J, Risques RA (2010) Telomere length, pre-eclampsia, and gestational diabetes. BMC Res Notes 3:113
Cross JA, Brennan C, Gray T et al (2009) Absence of telomere shortening and oxidative DNA damage in the young adult offspring of women with pre-gestational type 1 diabetes. Diabetologia 52:226–234
Zhang C, Hu FB, Olsen SF et al (2014) Rationale, design, and method of the Diabetes & Women’s health study--a study of long-term health implications of glucose intolerance in pregnancy and their determinants. Acta Obstet Gynecol Scand 93:1123–1130
Olsen SF, Houshmand-Oeregaard A, Granström C et al (2017) Diagnosing gestational diabetes mellitus in the Danish National Birth Cohort. Acta Obstet Gynecol Scand 96:563–569
Grunnet LG, Hansen S, Hjort L et al (2017) Adiposity, Dysmetabolic traits and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish National Birth Cohort. Diabetes Care 40:1746–1755
Tanner JM (1963) The regulation of human growth. Child Dev 34:817–847
Cawthon RM (2009) Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37:e21
Gilfillan C, Naidu P, Gunawan F, Hassan F, Tian P, Elwood N (2016) Leukocyte telomere length in the neonatal offspring of mothers with gestational and pre-gestational diabetes. Saretzki G, editor. PLoS One 11:e0163824
Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D (2015) Maternal and genetic factors determine early life telomere length. Proc Biol Sci 282:20142263
Holmes DK, Bellantuono I, Walkinshaw SA et al (2009) Telomere length dynamics differ in foetal and early post-natal human leukocytes in a longitudinal study. Biogerontology 10:279–284
Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS (2016) Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med 14:148
García-Calzón S, Moleres A, Marcos A et al (2014) Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: the EVASYON study. PLoS One 9:e89828
Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A (2011) The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal 15:3061–3100
Myatt L (2006) Placental adaptive responses and fetal programming. J Physiol 572:25–30
Nergadze SG, Farnung BO, Wischnewski H et al (2009) CpG-island promoters drive transcription of human telomeres. RNA 15:2186–2194
Li H, Simpson ER, Liu J-P (2010) Oestrogen, telomerase, ovarian ageing and cancer. Clin Exp Pharmacol Physiol 37:78–82
O’Tierney-Ginn P, Presley L, Minium J, Hauguel de Mouzon S, Catalano PM (2014) Sex-specific effects of maternal anthropometrics on body composition at birth. Am J Obstet Gynecol 211:292.e1–292.e9
Regnault N, Botton J, Heude B et al (2011) Higher cord C-peptide concentrations are associated with slower growth rate in the 1st year of life in girls but not in boys. Diabetes 60:2152–2159
Kubo A, Ferrara A, Windham GC et al (2014) Maternal hyperglycemia during pregnancy predicts adiposity of the offspring. Diabetes Care 37:2996–3002
Landon MB, Rice MM, Varner MW et al (2015) Mild gestational diabetes mellitus and long-term child health. Diabetes Care 38:445–452
Acknowledgements
We greatly appreciate all the children and their mothers who participated in the study. Additional GDM study team members include F. Bach Kampmann, M. Egholm, A.-C. Baun Thuesen, C. Møller Madsen, C. Fau Brinkløv and student assistants, who did tremendous work assisting in collecting data (Department of Endocrinology, Copenhagen University Hospital, Denmark). Additionally, we want to acknowledge the great collaboration with Staten’s Serum Institute DNBC study members S. Hansen, M. Strøm, K. Agerskov, C. Granström and A. Ahrendt in the planning and organisation of the GDM study (Centre for Fetal Programming, Statens Serum Institut, Copenhagen, Denmark) and C. Zhang for the collaboration on the Diabetes and Women’s Health (DWH) study (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA). We also kindly thank the clinics across Denmark’s regions for providing housing facilities for the clinical study.
Funding
This study was funded by the Danish Council for Strategic Research, the Innovation Fund Denmark (09-067124 and 11-115923), the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract #HHSN275201000020C), The European Commission (FP7-289346-EarlyNutrition), Rigshospitalet/The Copenhagen University Hospital, the faculty of Health and Medical Sciences, Copenhagen University and the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Contributions
LH, RV and RS designed the telomere study, and LGG, SFO and AV designed the GDM subcohort study. LH and LGG, together with the GDM/DNBC study team, collected the in vivo data. LH processed the blood samples and extracted DNA, and LH and RV performed the telomere length measurements. LH, RV and RS analysed the data, and LH, RV, LGG, DB, RS and AV interpreted the results of the experiments. LH wrote the manuscript, with contributions from RV, LGG, DB and RS. All co-authors revised the manuscript and approved the final version. AV is the guarantor of this work and takes responsibility for the contents and integrity of the data in this article.
Corresponding author
Ethics declarations
Duality of interest
AV is employed by AstraZeneca, Mölndal, Sweden and is shareholder of Novo Nordisk A/S. All other authors declare no potential conflict of interest relevant to this study.
Electronic supplementary material
ESM
(PDF 128 kb)
Rights and permissions
About this article
Cite this article
Hjort, L., Vryer, R., Grunnet, L.G. et al. Telomere length is reduced in 9- to 16-year-old girls exposed to gestational diabetes in utero. Diabetologia 61, 870–880 (2018). https://doi.org/10.1007/s00125-018-4549-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4549-7