Abstract
Aims/hypothesis
Stimulator of IFN genes (STING) is a central hub for cytosolic nucleic acid sensing and its activation results in upregulation of type I IFN production in innate immune cells. A type I IFN gene signature seen before the onset of type 1 diabetes has been suggested as a driver of disease initiation both in humans and in the NOD mouse model. A possible source of type I IFN is through activation of the STING pathway. Recent studies suggest that STING also has antiproliferative and proapoptotic functions in T cells that are independent of IFN. To investigate whether STING is involved in autoimmune diabetes, we examined the impact of genetic deletion of STING in NOD mice.
Methods
CRISPR/Cas9 gene editing was used to generate STING-deficient NOD mice. Quantitative real-time PCR was used to assess the level of type I IFN-regulated genes in islets from wild-type and STING-deficient NOD mice. The number of islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)206-214-specific CD8+ T cells was determined by magnetic bead-based MHC tetramer enrichment and flow cytometry. The incidence of spontaneous diabetes and diabetes after adoptive transfer of T cells was determined.
Results
STING deficiency partially attenuated the type I IFN gene signature in islets but did not suppress insulitis. STING-deficient NOD mice accumulated an increased number of IGRP206-214-specific CD8+ T cells (2878 ± 642 cells in NOD.STING−/− mice and 728.8 ± 196 cells in wild-type NOD mice) in peripheral lymphoid tissue, associated with a higher incidence of spontaneous diabetes (95.5% in NOD.STING−/− mice and 86.2% in wild-type NOD mice). Splenocytes from STING-deficient mice rapidly induced diabetes after adoptive transfer into irradiated NOD recipients (median survival 75 days for NOD recipients of NOD.STING−/− mouse splenocytes and 121 days for NOD recipients of NOD mouse splenocytes).
Conclusions/interpretation
Data suggest that sensing of endogenous nucleic acids through the STING pathway may be partially responsible for the type I IFN gene signature but not autoimmunity in NOD mice. Our results show that the STING pathway may play an unexpected intrinsic role in suppressing the number of diabetogenic T cells.
Graphical abstract

Similar content being viewed by others

Introduction
Type 1 diabetes results from destruction of pancreatic beta cells by autoreactive cytotoxic T cells specific for beta cell antigens. Environmental factors have been suggested to play a pivotal role in triggering of islet autoimmunity in individuals who are genetically more susceptible to developing type 1 diabetes [1, 2]. Despite many agents being proposed, the trigger for type 1 diabetes remains unclear because the disease process starts a long time before diagnosis. Viral infections have been suggested as exogenous triggers involved in the initiation of autoimmunity in susceptible individuals [3]. Virus infection can provoke a bystander immune response via pattern-recognition receptors recognising virus-derived foreign DNA as a pathogen-associated molecular pattern [4]. However, recent advances indicate that host-derived DNA can act as a danger-associated molecular pattern and also elicit a self-inflammatory response [5, 6]. In both cases, intracellular sensing of nucleic acid is required to stimulate these immune responses.
Stimulator of IFN genes (STING) is a central hub for cytosolic nucleic acid sensing in innate immunity and plays an important role in viral and tumour immunity [7]. STING is widely expressed in haematopoietic, endothelial and epithelial cells. A range of reports has focused on the function of STING in antigen-presenting cells during virus infection and tumour development [8]. In the cells responsible for innate immunity, pathogen- or tumour-delivered cytosolic nucleic acids stimulate a cyclic GMP-AMP synthase (cGAS)–cyclic GMP-AMP (cGAMP)–STING signalling cascade that activates both NF-κB and IFN regulatory factor 3 (IRF3) transcription pathways to induce expression of type I IFN [9]. Sensing of aberrant cytosolic DNA due to loss of function of DNA degradation enzymes, such as three-prime repair exonuclease-1 (TREX-1), can cause type I IFN-mediated autoinflammatory syndromes such as Aicardi–Gourtieres syndrome [10]. Gain-of-function mutations in STING can exacerbate inflammation, causing systemic vasculitis with lupus-like syndrome in humans that is characterised by increased type I IFNs and inflammatory cytokines (STING-associated vasculopathy with onset infancy [SAVI]) [11]. These reports suggest that dysregulated type I IFN production via the cGAS–cGAMP–STING pathway can contribute to the development of autoimmunity.
Recently, we generated NOD mice that were deficient for granzyme A, a trypsin-like serine protease that activates nucleases involved in the degradation of nucleic acids in the cytoplasm. These mice exhibited an increased diabetes incidence associated with accumulation of cytosolic DNA and increased type I IFN responses [12]. Transient elevation of a type I IFN-induced gene signature is a feature of early type 1 diabetes. An elevated type I IFN gene signature precedes autoimmunity in peripheral blood mononuclear cells of children genetically at risk of type 1 diabetes [13] and an increased type I IFN gene signature is evident in the islets of NOD mice at 3–4 weeks of age, before the infiltration of T cells [14, 15]. Type I IFN has therefore been postulated to be causative in type 1 diabetes pathogenesis by stimulating the immune system, beta cells and their milieu for initiation of islet autoimmunity. We hypothesise that the STING pathway is involved in sensing endogenous aberrant DNA, thereby increasing type I IFN production and type I IFN responses.
Although the role of STING in adaptive immunity is not fully elucidated, recent advances suggest that STING also has antiproliferative and proapoptotic functions in T cells that are T cell intrinsic and IFN independent [16,17,18,19,20]. A constitutively active STING mutation decreased proliferation and reduced T cell viability due to a disturbance of Ca2+ influx and increased endoplasmic reticulum stress upon T cell receptor stimulation in an IFN-independent manner [18, 19]. This recently described role for STING suggests that it may affect the progression of autoimmune diabetes where autoreactive T cells play a dominant role. To elucidate directly whether and how STING is involved in the pathogenesis of type 1 diabetes, we used CRISPR/Cas9 gene editing to generate STING-deficient NOD mice.
Methods
Ethics statement
All mice were maintained at St Vincent’s Institute, and all experiments were approved by the St Vincent’s Health animal ethics committee (AEC approval numbers 017/17 and 018/17).
Mice
A previously described method for CRISPR/Cas9 gene editing was adapted to target Sting1 (also known as Tmem173), the gene that encodes STING, in the NOD mouse strain [21]. NOD/ShiLtJ (The Jackson Laboratory, USA; https://www.jax.org/strain/001289) embryos were co-injected with Cas9 RNA and sgRNAs that were designed to delete the genomic region encompassing exons 3, 4 and 5 (sgRNA no. 1 5′GGATGACTAGTCAGGACCTT3′, sgRNA no. 2 5′TACTTGCGGTTGATCTTACC3′). G0 offspring were screened for gene mutations by amplifying the genomic regions encompassed by the sgRNAs using gene-specific primers. DNA sequencing of the PCR amplicons revealed a 962 bp deletion (em1: Chr18:35,738,680-35,739,641) and 956 bp deletion (em2: Chr18:35,738,683-35,739,638). Mice with either of these mutations were backcrossed to NOD/ShiLtJ mice, and subsequent offspring were intercrossed to establish two STING-deficient NOD mouse lines: NOD.Sting1em1/em1 and NOD.Sting1em2/em2 (henceforth, both lines referred to as NOD.STING−/−). NOD.Cg-Tg(TcraTcrbNY8.3)1Pesa transgenic NOD mice (NOD8.3) [22] were crossed with NOD.STING−/− mice to produce STING-deficient NOD8.3 mice (NOD8.3/STING−/−). Genotyping of Sting1 alleles and the 8.3 TCR transgene was performed using DNA isolated from tail biopsies and standard PCR/gel electrophoresis methods. Sting1 alleles were detected using two different primer combinations: primer set 1 for simultaneous detection of the deletion alleles and wild-type allele (forward GGGCGTCTCCTTGAGGTGTA; reverse AACTGTATGGCAGACATGGAAATAC) and primer set 2 for detection of the wild-type allele (forward CCTCCAAAACACTGCTGACA; reverse CACCCTTCACACAGACAGGA).
Diabetes, insulitis and insulin autoantibodies
Female and male mice were monitored for spontaneous diabetes for 300 days. Mice with two consecutive blood glucose measurements of more than 15 mmol/l were diagnosed diabetic. For histology, tissues were fixed with 10% vol./vol. neutral buffered formalin. Pancreatic sections from three levels (200 μm apart) of paraffin-embedded samples were analysed by staining with H&E. Two independent investigators scored the severity of insulitis in a blinded manner as follows: 0, no lymphocytic infiltration; 1, peri-islet infiltration; 2, extensive peri-islet infiltration; 3, intra-islet infiltration; and 4, extensive intra-islet infiltration or total beta cell loss. Insulitis scores were calculated by the method previously described [23]. Insulin autoantibodies (IAA) were measured by non-competitive ELISA assay using 96-well plates as previously described [24]. Each sample was tested with and without plate-bound insulin in duplicate and the absorbance value at 450 nm was calculated by subtracting the absorbance of the sample without plate-bound insulin from the absorbance of the sample with plate-bound insulin.
Islet isolation
Islets were isolated using collagenase P (Roche, Basel, Switzerland) and Histopaque-1077 density gradients (Sigma-Aldrich, USA) as previously described [25].
Adoptive transfer of carboxyfluorescein succinimidyl ester-labelled 8.3 T cells
CD8+ T cells isolated from NOD8.3 or NOD8.3/STING−/− mice were labelled with carboxyfluorescein succinimidyl ester (CFSE) and suspended at a concentration of 2.5 × 107 cells/ml in PBS as previously described [23]. CFSE-labelled T cells (0.5 × 107 cells) were intravenously injected into the tail veins of recipient mice. The pancreatic lymph nodes (PLNs) and islets were harvested from the recipient mice 5 days after the transfer. Proliferation of CFSE-labelled cells was assessed by flow cytometry as described below.
To obtain dendritic cells, spleen was digested with collagenase (1 mg/ml) and DNAse (0.02 mg/ml) and then treated with EDTA. Non-dendritic cells were magnetically depleted then dendritic cells were enriched using anti-CD11c (Miltenyi Biotech, Germany). CFSE-labelled CD8+ T cells (1 × 105 cells/well) were incubated with islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)206-214 peptide (1 μmol/l)-coated dendritic cells (1 × 104 cells/well) in quadruplicate. Proliferation of CFSE-labelled T cells was measured by flow cytometry after 3 days of culture.
Adoptive transfer into irradiated NOD mice
NOD mice, aged 8–10 weeks, received 650 rad-irradiation twice, and 20 × 106 splenocytes from non-diabetic 12- to 15-week-old NOD or NOD.STING−/− donors were transferred into the irradiated mice intravenously. The recipient mice were monitored for diabetes until 150 days after the transfer.
Real-time PCR
Bone marrow cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin, 292 mg/ml l-glutamine (Gibco, USA) and 20% L929 conditioned medium. After incubation overnight, semi-adherent cells were collected in fresh complete DMEM and incubated for 4 days to obtain a population of differentiated macrophages. Bone marrow-derived macrophages (BMDMs; 1.5 × 105) generated from NOD and NOD.STING−/− mice were stimulated with STING agonist (5,6-dimethylxanthenone-4-acetic acid [DMXAA], 50 ng/μl) for 2 h. Total RNA was extracted using ISOLATE RNA Micro Kit (BIOLINE, Luckenwalde, Germany) and cDNA was synthesised using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Quantitative real-time PCR analysis was performed using a LightCycler 480 (Roche) and TaqMan gene expression assays for Mx1 (probe ID: Mm00487796_m1), Ifit1 (probe ID: Mm00515153_m1), Oas1a (probe ID: Mm00836412_m1), Oas1b (probe ID: Mm00449297_m1), Isg15 (probe ID: Mm01705338_s1), Cxcl10 (probe ID: Mm00445235_m1), Ifnb1 (probe ID: Mm00439552_s1) and Actb (probe ID: Mm002619580_g1) (Applied Biosystems, Foster City, CA, USA). Threshold cycle (Ct) values were normalised to the Ct values of actin in individual samples and expressed as ΔCt. Relative expression levels were then calculated as \( {2}^{-\Delta {\mathrm{C}}_{\mathrm{t}}} \).
Flow cytometry
Single-cell suspensions were prepared from spleen, PLN and inguinal lymph node (ILN) by mechanical disruption using the flat end of a syringe plunger. Disrupted cells were passed through a 70 μm filter-mesh and treated with ammonium chloride buffer to lyse red blood cells. For surface staining, cells were stained with the corresponding fluorescence-labelled antibodies. The following antibodies (clone, catalogue number) were from Biolegend, USA: CD45 (30F11,103132), CD3e (145-2C11, 100341), CD4 (GK1.5,100408), CD8 (53-6.7,100722), CD19 (6D5,115529), CD25 (3C7,101910), plasmacytoid dendritic cell antigen-1 (PDCA-1; 927,127023), CD44 (IM7,103030), CD62L (MEL-14,104408), CD40 (1C10,102805), CD80 (16-10A1,104733), programmed cell death protein-1 (PD-1; 29F.1A12,135220) and T cell immunoreceptor with Ig and ITIM domains (TIGIT; VSIG9,142110). CD45R (RA3-6B2,48-0452-82), CD11c (N418,48-0114-82), CD11b (M1/70,48-0112-82) and lymphocyte antigen 6 complex locus G6D (Ly-6G; RB6-8C5,48-5931-82) antibodies were purchased from eBiosciences, USA. CD86 (GL1,563055), MHC I (SF1-1.1,553564), MHC II (OX-6, 554928) were from BD Biosciences, USA. T cell immunoglobulin and mucin domain containing protein 3 (TIM-3; 215008,FAB1529G) was obtained from R&D systems, USA. For intracellular staining of STING and forkhead box P3 (FOXP3), cells were fixed with Foxp3/Transcription Factor Staining Buffer Set (eBioscience, USA). For intracellular cytokine staining, cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin (Sigma, St Louis, MO, USA) in the presence of Golgi Plug (BD Biosciences, USA) for 4 h and then fixed with Cytofix/Cytoperm solution (BD Biosciences). The Cytofix/Cytoperm solution was also used for staining granyzme B on CFSE-labelled 8.3 T cells without re-stimulation of the cells. After the fixation and permeabilisation, cells were stained for intracellular antigens of interest; STING (clone 41), FOXP3 (FJK-16S), IFNγ (XMG1.2) and granzyme B (NGZB). Beta cells were identified by their autofluorescence as previously described [25]. IGRP-specific CD8+ T cells were assessed using H2-Kd tetramer with magnetic bead-based enrichment as described previously [24]. Briefly, cells from peripheral lymphoid organs (spleen, ILNs, and mesenteric lymph nodes) were stained with phycoerythrin-conjugated IGRP206-214 (VYLKTNVFL) H2-Kd tetramer (ImmunoID, Parkville, Australia) and phycoerythrin-conjugated I-Ag7-INSB10-23 (HLVERLYLVCGGEG) tetramer (NIH tetramer core, Georgia, USA). Cells were stained with anti-phycoerythrin microbeads (Miltenyi Biotec) and separated using the AutoMACS pro separator (Miltenyi Biotec). Subsequently, the cells were stained for surface molecules and analysed by flow cytometry. H2-Kd-TUM (KYQAVTTTL) tetramers were used to determine background staining. Data were collected on a BD LSRFortessa (BD Biosciences) and analysed using FlowJo analysis software version 10.7.1 (FlowJo, Ashland, OR, USA).
Statistics
Statistical analysis for group differences was performed using the unpaired Student’s t test or a two-way ANOVA and statistical analysis on Kaplan–Meier estimates were performed using the logrank test by GraphPad Prism software version 8 (GraphPad, USA). All data shown as bar graphs are represented as mean ± SD. p < 0.05 was considered significant.
Results
Generation of STING-deficient NOD mice
To determine whether STING is involved in the pathogenesis of type 1 diabetes, we used CRISPR/Cas9 gene editing in NOD mice to disrupt Sting1, the gene encoding STING. PCR-based genotyping (Fig. 1a) and sequence analysis (not shown) were used to identify and establish NOD.STING−/− mice that contained genomic deletions encompassing exon 3, 4 and 5 of Sting1.
Genetic, protein and functional analysis confirm STING deficiency in NOD.STING−/− mice. (a) Genomic DNA was analysed by PCR-based genotyping and agarose gel electrophoresis. Two sets of primers were used to detect the presence or absence of the genomic interval encompassing exon 3, 4 and 5 of Sting1. Black arrows indicate the PCR products of the wild-type allele and the white arrow indicates the mutant allele, in which the genomic interval has been deleted. The wild-type product is not efficiently amplified in the presence of the deletion allele for primer set 1, hence the design and use of primer set 2. (b) Spleen cells were fixed, permeabilised and stained with anti-STING antibody. Representative flow cytometry plots of NOD and NOD.STING−/− cells are shown together with pooled data showing the proportion of STING-positive spleen cells. Data are mean ± SD for NOD (n=5), NOD.STING−/− (em1, n=3; em2, n=3), ***p<0.0001 for NOD.STING groups vs NOD (one-way ANOVA with Dunnett’s post hoc test for multiple comparisons). (c, d) BMDMs were generated from wild-type or NOD.STING−/− mice and stimulated with the STING agonist DMXAA. Relative expression levels (\( {2}^{-\Delta {\mathrm{C}}_{\mathrm{t}}} \)) of Cxcl10 (c) and Ifnb1 transcripts (d) were measured by quantitative real-time PCR. Data show mean ± SD for individual NOD (n=2) and NOD.STING−/− (n=3) mice. NC, negative control; WT, wild-type
We confirmed the lack of STING protein in NOD.STING−/− mouse spleen cells by flow cytometry (Fig. 1b). To verify functional deletion of STING, we stimulated BMDMs generated from NOD and NOD.STING−/− mice with a STING agonist (DMXAA) that triggers activation of the cGAS–cGAMP–STING pathway to upregulate Ifnb1 and Cxcl10 [26]. Expression of Ifnb1 and Cxcl10 was upregulated in BMDMs derived from wild-type NOD mice after treatment with DMXAA but was not detected in NOD.STING−/− BMDMs (Fig. 1c,d).
STING deficiency partially attenuates expression of type I IFN-regulated genes in islets
A type I IFN signature has been observed in the early phase of islet autoimmunity in human type 1 diabetes and in NOD mice [13,14,15, 27, 28]. STING is a central hub in nucleic acid sensing resulting in production of type I IFN, therefore, we examined the expression of type I IFN-regulated genes in islets of NOD.STING−/− mice. If DNA sensing via STING is important in driving the type I IFN gene signature in NOD mice, we would expect the gene signature to be reduced in NOD.STING−/− mice. We detected a lower expression of Oas1a, Oas1b and Isg15 in islets isolated from 20-day-old NOD.STING−/− mice compared with wild-type NOD mice, while other IFN-regulated genes, including Ifit1, Mx1 and Cxcl10, were unaffected by loss of STING (Fig. 2a–f). Gene expression was similar when comparing islets from wild-type and STING−/− mice aged 100 days, with the exception of Mx1, which was slightly reduced in the NOD.STING−/− islets (Fig. 2g–l). These data suggest that STING-dependent nucleic acid sensing may be partly involved in increasing type I IFN gene expression in the islets of young NOD mice. However, gene expression was not completely abolished, as in NOD mice lacking Ifnar1 [15], indicating the presence of other stimulators of type I IFN in islets.
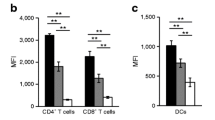
A partial decrease in islet type I IFN gene signature does not affect antigen-presenting cell phenotypes in NOD.STING−/− mice. (a–l) Total RNA was collected from the islets of NOD and NOD.STING−/− mice at 20 days of age (n=5 mice/genotype) (a–f) and 100 days of age (n=6 mice/genotype) (g–l). Expression of type I IFN-regulated genes IfIt1, Oas1a, Oas1b, Isg15, Mx1and Cxcl10 was evaluated by quantitative real-time PCR. Data show mean ± SD of ΔCt values, with symbols showing individual mice, each performed in triplicate. For NOD.STING−/− vs NOD: *p=0.041 for Mx1 (k); **p=0.0036 for Oas1a (b), p=0.0024 for Oas1b (c) and p=0.0017 for Isg15 (d) (Student’s t test). (m, n) MHC class I expression on beta cells from 120-day-old NOD and NOD.STING−/− mice as assessed by flow cytometry. Viable beta cells were identified by their high autofluorescence as described in the Methods. Representative histograms of MHC class I (m) and quantification of mean fluorescence intensity of MHC class I (n) on beta cells isolated from NOD (n=6) and NOD.STING−/− (n=6) mice. Data show individual mice and the mean. No significant difference was noted between the mouse groups (Student’s t test). (o, p) Infiltrating leucocytes (PI−CD45+) from islets were analysed by flow cytometry for the expression of CD40, CD80, CD86 and MHC class II on conventional dendritic cells (CD3−CD19−PDCA-1−CD11c+). Representative histograms (o) and quantification of mean fluorescence intensity of CD40, CD80, CD86 and MHC class II (p) on cDC in islets of NOD (n=5) and NOD.STING−/− (n=6) mice. Data show individual mice and the mean. No significant difference was noted between the mouse groups (two-way ANOVA with Sidak’s multiple comparison test). (q) The proportion of CD45+ cells that were conventional dendritic cells (CD3−CD19−PDCA-1−CD11c+) and plasmacytoid dendritic cells (CD3−CD19−PDCA-1+CD11c+) in the islets of 120-day-old female NOD (n=6) and NOD.STING−/− (n=6) mice. Data show mean and individual mice. No significant difference was noted between the mouse groups (two-way ANOVA). cDC, conventional dendritic cells; MFI, mean fluorescence intensity; pDC, plasmacytoid dendritic cells; PI, propidium iodide
MHC class I hyperexpression on beta cells is a hallmark of type 1 diabetes [29] and type I IFN can upregulate MHC class I expression on beta cells in vitro [15]. We observed equivalent expression of MHC class I on beta cells in NOD.STING−/− mice compared with NOD mice at 120 days of age (Fig. 2m,n and electronic supplementary material [ESM] Fig. 1a). This is consistent with our previous finding that IFNγ, and not type I IFN, primarily regulates MHC class I on beta cells in vivo in NOD mice [15, 30].
Conventional dendritic cells play a major role in presenting autoantigens to diabetogenic T cells, and local type I IFN production in inflamed islets could affect their maturation. No differences in the expression of CD40, CD80, CD86 and MHC class II on conventional dendritic cells were detected in NOD.STING−/− and NOD mice at 120 days of age (Fig. 2o,p and ESM Fig. 1b) and there was no difference in the number of conventional or plasmacytoid dendritic cells (Fig. 2q). STING−/− antigen-presenting cells efficiently stimulated islet antigen-specific CD8+ T cells in vitro (ESM Fig. 2a). These data indicate that STING deficiency partially decreases the type I IFN signature in islets but does not attenuate antigen-presenting cell phenotype or function.
STING deficiency promotes spontaneous diabetes in NOD mice
We next determined whether STING deficiency impacts spontaneous diabetes. Female NOD.STING−/− mice (from the em2 line) had a significantly higher incidence of spontaneous diabetes than female wild-type NOD mice (Fig. 3a). The cumulative incidence of spontaneous diabetes at 300 days of age was 95.5% in the NOD.STING−/− em1 line, 93.7% in the NOD.STING−/− em2 line and 86.2% in wild-type NOD mice. Diabetes incidence was also greater in male mice, with an incidence of 69.2% in the NOD.STING−/− em2 line and 51.2% in NOD mice (p = 0.02, logrank test). To confirm whether STING deficiency enhances the autoimmune pathogenicity of immune cells, splenocytes from NOD and NOD.STING−/− mice were adoptively transferred into irradiated wild-type NOD mice. STING-deficient splenocytes transferred diabetes earlier than wild-type NOD splenocytes (median survival 75 days for NOD recipients of NOD.STING−/− mouse splenocytes and 121 days for NOD recipients of NOD mouse splenocytes) (Fig. 3b).
STING deficiency increases the incidence of diabetes in NOD mice with equivalent severity of insulitis. (a) Cumulative incidence of spontaneous diabetes in female wild-type NOD mice (n=55) compared with STING-deficient NOD mice (em1, n=49; em2, n=54); p=0.045 (logrank test with correction for multiple testing). (b) Cumulative incidence of diabetes after adoptive transfer of splenocytes from non-diabetic 12- to 15-week-old NOD or NOD.STING−/− mice into 8- to 10-week-old recipient irradiated NOD mice (n=20 recipients of NOD cells and n=23 recipients of NOD.STING−/− cells pooled from n=3 independent experiments); p=0.0006 (logrank test). (c) Representative H&E-stained sections of pancreas harvested from 100-day-old female NOD and NOD.STING−/− mice. Arrows show insulitis. Magnification ×100; scale bar, 100 μm. (d) Combined insulitis scores of female NOD and NOD.STING−/− mice at 30, 100 and 150 days of age. The insulitis score for each mouse was calculated as described in the Methods. Data show the scores of three levels of the pancreas from individual mice and mean of n=5–15/group; no significant difference between groups was noted (two-way ANOVA with Sidak’s multiple comparison test). (e) The percentage of CD45+ cells in islets cells isolated from NOD and NOD.STING−/− mice at 84 and 120 days of age as determined by flow cytometry. Data are presented for individual mice and as mean of n=6/group performed in three independent experiments; no significant difference was noted between groups (two-way ANOVA with Sidak’s multiple comparison test). (f, g) The proportion of CD3+CD45+ cells (f) and CD4+CD3+and CD8+CD3+ T cells (g) in the islets of 120-day-old female NOD (n=6) and NOD.STING−/− (n=5 or 6) mice. Data are presented as means and for individual mice pooled from three independent experiments. No significant difference was noted between groups (Student’s t test). (h) Absorbance (at 450 nm) for IAA of serum from individual NOD (n=15) and NOD.STING−/− (n=10) mice at 84 days of age measured by ELISA. Each sample was assayed in duplicate. Data are presented for individual mice and as mean. No significant difference was noted between groups (Student’s t test)
We assessed the level of insulitis and development of IAA. Despite the increased diabetes incidence, NOD.STING−/− mice displayed a similar severity of insulitis at 30, 100 and 150 days of age compared with wild-type NOD mice (Fig. 3c,d). Flow cytometric analysis confirmed that NOD.STING−/− mice had a similar frequency of CD45+ cells in the islets at 84 and 120 days of age, and the proportion of CD3+CD45+ cells, CD4+CD3+ and CD8+CD3+ cells was equivalent at 120 days of age, when compared with wild-type NOD mice (Fig. 3e–g). There was no significant difference in levels of IAA when comparing NOD and NOD.STING−/− mice at 84 days of age (Fig. 3h), indicating normal B cell autoantibody-producing function.
STING deficiency promotes generation of autoreactive T cells in NOD mice
Autoimmune diabetes in NOD mice results from autoreactive T cell-mediated destruction of beta cells. IGRP-specific CD8+ T cells comprise a large proportion of the antigen-specific T cells in NOD mice and have also been detected in human type 1 diabetes [31, 32]. A recent report suggested that STING has an intrinsic role in repression of T cell proliferation [18]. Therefore, we hypothesised that STING deficiency may promote diabetes development by promoting the expansion of autoreactive T cells. To test this hypothesis, we tracked the number of IGRP206-214-specific CD8+ T cells in the peripheral lymphoid tissues in NOD mice using MHC tetramer enrichment [31]. NOD.STING−/− mice had significantly increased numbers of IGRP206-214 tetramer+ CD8+ T cells (2878 ± 642 cells in NOD.STING−/− mice and 728.8 ± 196 cells in wild-type NOD mice) in their peripheral lymphoid tissues compared with wild-type NOD mice (Fig. 4a,b). The IGRP206-214 tetramer+ CD8+ T cells in peripheral lymphoid tissues of NOD.STING−/− mice had a memory phenotype with high levels of CD44 expression, while the number of the CD62L-high naive cells was equivalent to that of wild-type NOD mice (Fig. 4c,d). We saw no difference in the number of INSB10-23-specific CD4+ T cells in NOD.STING−/− and wild-type mouse tissues (Fig. 4e).
Increased autoreactive T cells in NOD.STING−/− mice. (a–d) IGRP206-214 tetramer, CD8, CD44 and CD62L staining in peripheral lymphoid organs of 84-day-old NOD and NOD.STING−/− mice. Representative staining of IGRP tetramer+CD8+ and CD44+IGRP tetramer+ cells are shown (a), with quantification of IGRP206-214 tetramer+ CD8+ T cells (b), and CD44+ (c) and CD62L+ IGRP206-214 tetramer+ CD8+ T cells (d). Data show individual values and mean for NOD (n=17−25) and NOD.STING−/− (n=10−28) mice. **p=0.001 and ***p=0.0006 (Student’s t test). (e) Quantification of INSB10-23 tetramer+CD4+ T cells in peripheral lymphoid organs of 84- to 120-day-old NOD and NOD.STING−/− mice. Data show individual values and mean for NOD (n=12) and NOD.STING−/− (n=13) mice. No significant difference was noted between groups (Student’s t test). (f) Mean fluorescence intensity of granzyme B expression on CD8+ T cells from ILNs, PLNs and islets of wild-type NOD8.3 and NOD8.3/STING−/− mice on day 5 after adoptive transfer into NOD or NOD.STING−/− recipient mice. No significant difference was noted between NOD8.3 and NOD8.3/STING−/− in each tissue or recipient (two-way ANOVA). (g) The proportion of IFNγ-producing CD4+ and CD8+ T cells in islets from NOD (n=5) and NOD.STING−/− (n=4) mice after stimulation with phorbol 12-myristate 13-acetate and ionomycin. Data are shown for individual mice and as mean. No significant difference was noted between groups (two-way ANOVA). (h) Mean fluorescence intensity of PD-1, TIGIT and TIM-3 on CD8+ T cells infiltrating into islets of 120-day-old NOD (n=6) and NOD.STING−/− (n=5) mice. Data are shown for individual mice and as mean. No significant difference was noted between groups (two-way ANOVA). MFI, mean fluorescence intensity
To test the function of STING−/− cells, CD8+ T cells were transferred from NOD8.3 or NOD8.3/STING−/− mice, which have >90% CD8+ T cells that express the 8.3 TCR recognising IGRP206-214. Equivalent levels of the effector molecule granzyme B were expressed in wild-type and STING−/− CD8+ T cells (Fig. 4f). STING deficiency did not affect the proportion of T cell subsets including naive T cells, memory T cells and regulatory T cells. The total number of mononuclear cells in lymphoid tissues was also unaffected (ESM Fig. 2b–e). The proportion of IFNγ-producing CD4+ and CD8+ T cells in islets and the expression of exhaustion markers PD-1, TIGIT and TIM-3 in islet-infiltrating CD8+ T cells was the same for NOD.STING−/− and NOD mice at 120 days of age (Fig. 4g,h). These data suggest that STING deficiency increases the number of autoreactive memory CD8+ T cells but does not affect their activation and effector function and does not affect the number of autoreactive CD4+ T cells.
Discussion
In individuals born with genetic susceptibility, autoimmune disease such as type 1 diabetes can be triggered by environmental factors, such as viral and bacterial infection and early exposure to dietary constituents [1, 2]. Because expression of type I IFN-regulated genes is increased in the early phase of autoimmunity in NOD mice and individuals with type 1 diabetes [13,14,15], it has been suggested that type I IFN may change the islet microenvironment, allowing a break in tolerance in genetically susceptible individuals. The trigger for increased expression of type I IFN-regulated genes remains unknown. Using CRISPR/Cas9 gene editing, we generated NOD mice deficient in the nucleic acid adaptor protein STING to test the hypothesis that preventing sensing of endogenous nucleic acids would reduce the type I IFN gene signature in the islets of NOD mice.
We saw transient partial reduction in the type I IFN gene signature in the islets of STING-deficient mice around the onset of autoimmunity, suggesting that nucleic acid sensing via the cGAS–STING pathway may only partially account for the gene signature previously reported. Recent discoveries have revealed monogenic mutations that enhance endogenous nucleic acid sensing via pattern-recognition receptors and cause autoinflammatory syndromes as a result of constitutive type I IFN production and an inflammatory self-directed immune response [33]. In autoimmune disease, genetic susceptibility loci for systemic lupus erythematosus (SLE) were identified that encompass genes coding for the nucleic acid sensors Toll-like receptor (TLR) 7 and melanoma differentiation associated gene 5 (MDA5) [34, 35], and genes involved in the regulation of endogenous nucleic acid sensing such as DNase1, DNase1L3, RNase H2 and TREX1 [36,37,38,39]. Polymorphisms in IFIH1, the gene encoding the double-stranded RNA sensor MDA5, are associated with type 1 diabetes [40]. Haplo-deficiency of MDA5, a model for the type 1 diabetes-associated polymorphism, attenuated insulitis and abolished diabetes in a virus-triggered model for autoimmune diabetes [41]. However, we identified no inhibitory effect of STING deficiency on the progression of autoimmune diabetes and immune homeostasis in NOD mice. These data suggest that sensing of endogenous nucleic acids via the cGAS–STING pathway is dispensable for the development of spontaneous autoimmune diabetes in NOD mice. The IFN gene signature was not completely abolished in the islets of STING-deficient mice, which suggests that other pathways for nucleic acid sensing, such as through TLR7, TLR9, myeloid differentiation primary response 88 (MyD88) or MDA5, might be relevant to the signature as their genetic deletion resulted in protection from autoimmune diabetes in NOD mice [41, 42]. Despite evidence for an increase in a type I IFN gene signature in humans and NOD mice, deficiency of receptors for type I IFN (IFNAR1) either resulted in no effect or partial protection from diabetes in female NOD mice depending on the animal facility [15, 43]. This is consistent with our current data suggesting that the type I IFN gene signature is not directly tied to insulitis development. While diabetes in NOD mice is not dependent on virus infection, there is evidence for a possible viral aetiology in human type 1 diabetes [1, 2]. STING–cGAS signalling is required for clearance of DNA virus infections [44], and its deficiency in NOD mice is also likely to affect such infection models.
Contrary to our initial hypothesis, we observed a slightly increased diabetes incidence in STING-deficient NOD mice. A previous study also identified a paradoxical role for STING in controlling autoimmunity. Sharma et al observed an increase in SLE-like symptoms, including increased autoantibodies, a robust type I IFN gene signature and increased immune activation, in STING-deficient MRL.lpr mice [45]. This negative regulatory role of STING was independent of the transcription factor IRF3. Our findings are also consistent with a study showing reduced frequency of diabetes in NOD mice treated with cGAMP, although the activation of STING by cGAMP was not directly shown in that study [46]. Despite reduced frequency of diabetes, type I IFN was increased in the serum, and inflammatory cytokines (TNF and IL-6) were increased in the spleen of cGAMP-treated NOD mice. These studies and ours indicate a possible role for STING in controlling autoimmunity that is independent of its DNA sensing function and of type I IFN production.
We detected an increase in autoreactive CD8+ T cells in STING-deficient NOD mice and a more potent capacity of STING-deficient T cells to transfer diabetes to immunodeficient mice. Although our data do not directly rule out a role for innate immune cells, the expansion of autoreactive CD8+ T cells is likely to be intrinsic to T cells as levels of MHC class I on beta cells, maturation markers on conventional dendritic cells, antigen presentation by dendritic cells and B cell number were equivalent in wild-type and NOD.STING mice. A previous study demonstrated a role for STING in promoting antibody responses [47]; however, there were normal levels of IAA in STING-deficient NOD mice. We did not directly observe increased capacity of early-phase proliferation of STING-deficient T cells in our NOD.STING−/− mice in vitro or of transferred STING-deficient NOD8.3 T cells (data not shown). Therefore, in NOD mice with a polyclonal repertoire of T cells and chronic inflammation, STING may restrain proliferation or promote apoptosis of autoreactive CD8+ T cells. While the mechanism remains unclear, others have also revealed antiproliferative and proapoptotic functions of STING in T cells [16,17,18,19,20]. One report documented decreased proliferation of CD4+ T cells transduced with a constitutive gain-of-function STING mutation [18]. Another recent report uncovered that a constitutively activating STING mutation reduced their viability due to a disturbance of Ca2+ influx and increased endoplasmic reticulum stress upon T cell receptor stimulation [19]. These previous findings are consistent with our data showing increased number of IGRP-specific cells and increased capacity of STING-deficient cells to transfer diabetes. However, we did not see any impact of STING deficiency on CD4+ T cells, with similar numbers of insulin-specific CD4+ T cells, similar IFNγ production and normal IAA, indicating normal interaction between CD4+ T cells and B cells. We are still gaining new insights into the role of STING in different cell types, including CD8+ T cells, and different disease settings. Our data suggest that STING, while primarily contributing to sensing self and non-self DNA, may contribute to autoimmunity by controlling autoreactive CD8+ T cell proliferation or apoptosis.
Current research is focused on targeting the cGAS–STING pathway for infectious diseases, cancer, autoinflammatory diseases and autoimmune diseases [48]. STING agonism has the potential to be used as an immunomodulatory therapy for cancer and a vaccine adjuvant for chronic viral or bacterial infections. Alternatively, STING antagonism has been suggested as therapy for autoinflammatory diseases such as SAVI, Aicardi-Goutieres Syndrome, SLE and metastatic cancer. Excessive STING agonism may induce apoptosis and suppress the expansion of tumour antigen-specific T cells, as previously shown [49]. Our findings of expanded autoreactive T cells and increased diabetes incidence in STING-deficient NOD mice suggest that systemic STING antagonism could trigger autoimmunity in individuals susceptible for T cell-driven autoimmune diseases such as type 1 diabetes.
In conclusion, we find that the innate immune adaptor STING is not primarily responsible for the type I IFN signature in islets during the early stages of pathogenesis but may have an intrinsic role to suppress the expansion of autoreactive T cells in NOD mice.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Abbreviations
- BMDM:
-
Bone marrow-derived macrophage
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- cGAMP:
-
Cyclic GMP-AMP
- cGAS:
-
Cyclic GMP-AMP synthase
- DMXAA:
-
5,6-Dimethylxanthenone-4-acetic acid
- FOXP3:
-
Forkhead box P3
- IAA:
-
Insulin autoantibodies
- IGRP:
-
Islet-specific glucose-6-phosphatase catalytic subunit-related protein
- ILN:
-
Inguinal lymph node
- IRF3:
-
IFN regulatory factor 3
- Ly-6G:
-
Lymphocyte antigen 6 complex locus G6D
- MDA5:
-
Melanoma differentiation associated gene 5
- PD-1:
-
Programmed cell death protein-1
- PDCA-1:
-
Plasmacytoid dendritic cell antigen-1
- PLN:
-
Pancreatic lymph node
- SAVI:
-
STING-associated vasculopathy with onset infancy
- SLE:
-
Systemic lupus erythematosus
- STING:
-
Stimulator of IFN genes
- TLR:
-
Toll-like receptor
- TREX-1:
-
Three-prime repair exonuclease-1
- TIGIT:
-
T cell immunoreceptor with Ig and ITIM domains
- TIM-3:
-
T cell immunoglobulin and mucin domain containing protein 3
References
Craig ME, Kim KW, Isaacs SR et al (2019) Early-life factors contributing to type 1 diabetes. Diabetologia 62(10):1823–1834. https://doi.org/10.1007/s00125-019-4942-x
Rewers M, Ludvigsson J (2016) Environmental risk factors for type 1 diabetes. Lancet 387(10035):2340–2348. https://doi.org/10.1016/S0140-6736(16)30507-4
van der Werf N, Kroese FG, Rozing J, Hillebrands JL (2007) Viral infections as potential triggers of type 1 diabetes. Diabetes Metab Res Rev 23(3):169–183. https://doi.org/10.1002/dmrr.695
Pane JA, Coulson BS (2015) Lessons from the mouse: potential contribution of bystander lymphocyte activation by viruses to human type 1 diabetes. Diabetologia 58(6):1149–1159. https://doi.org/10.1007/s00125-015-3562-3
Ablasser A, Chen ZJ (2019) cGAS in action: Expanding roles in immunity and inflammation. Science 363(6431):eaat8657. https://doi.org/10.1126/science.aat8657
Crowl JT, Gray EE, Pestal K, Volkman HE, Stetson DB (2017) Intracellular Nucleic Acid Detection in Autoimmunity. Annu Rev Immunol 35:313–336. https://doi.org/10.1146/annurev-immunol-051116-052331
Barber GN (2015) STING: infection, inflammation and cancer. Nat Rev Immunol 15(12):760–770. https://doi.org/10.1038/nri3921
Corrales L, McWhirter SM, Dubensky TW Jr, Gajewski TF (2016) The host STING pathway at the interface of cancer and immunity. J Clin Invest 126(7):2404–2411. https://doi.org/10.1172/JCI86892
Chen Q, Sun L, Chen ZJ (2016) Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17(10):1142–1149. https://doi.org/10.1038/ni.3558
Crow YJ, Hayward BE, Parmar R et al (2006) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet 38(8):917–920. https://doi.org/10.1038/ng1845
Liu Y, Jesus AA, Marrero B et al (2014) Activated STING in a vascular and pulmonary syndrome. N Engl J Med 371(6):507–518. https://doi.org/10.1056/NEJMoa1312625
Mollah ZUA, Quah HS, Graham KL et al (2017) Granzyme A Deficiency Breaks Immune Tolerance and Promotes Autoimmune Diabetes Through a Type I Interferon-Dependent Pathway. Diabetes 66(12):3041–3050. https://doi.org/10.2337/db17-0517
Ferreira RC, Guo H, Coulson RM et al (2014) A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 63(7):2538–2550. https://doi.org/10.2337/db13-1777
Diana J, Simoni Y, Furio L et al (2013) Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 19(1):65–73. https://doi.org/10.1038/nm.3042
Quah HS, Miranda-Hernandez S, Khoo A et al (2014) Deficiency in type I interferon signaling prevents the early interferon-induced gene signature in pancreatic islets but not type 1 diabetes in NOD mice. Diabetes 63(3):1032–1040. https://doi.org/10.2337/db13-1210
Larkin B, Ilyukha V, Sorokin M, Buzdin A, Vannier E, Poltorak A (2017) Cutting Edge: Activation of STING in T Cells Induces Type I IFN Responses and Cell Death. J Immunol 199(2):397–402. https://doi.org/10.4049/jimmunol.1601999
Gulen MF, Koch U, Haag SM et al (2017) Signalling strength determines proapoptotic functions of STING. Nat Commun 8(1):427. https://doi.org/10.1038/s41467-017-00573-w
Cerboni S, Jeremiah N, Gentili M et al (2017) Intrinsic antiproliferative activity of the innate sensor STING in T lymphocytes. J Exp Med 214(6):1769–1785. https://doi.org/10.1084/jem.20161674
Wu J, Chen YJ, Dobbs N et al (2019) STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med 216(4):867–883. https://doi.org/10.1084/jem.20182192
Wu J, Dobbs N, Yang K, Yan N (2020) Interferon-Independent Activities of Mammalian STING Mediate Antiviral Response and Tumor Immune Evasion. Immunity 53(1):115–126 e115. https://doi.org/10.1016/j.immuni.2020.06.009
Brandl C, Ortiz O, Rottig B, Wefers B, Wurst W, Kuhn R (2015) Creation of targeted genomic deletions using TALEN or CRISPR/Cas nuclease pairs in one-cell mouse embryos. FEBS Open Bio 5:26–35. https://doi.org/10.1016/j.fob.2014.11.009
Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P (1997) Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med 186(10):1663–1676. https://doi.org/10.1084/jem.186.10.1663
Mollah ZU, Graham KL, Krishnamurthy B et al (2012) Granzyme B is dispensable in the development of diabetes in non-obese diabetic mice. PLoS One 7(7):e40357. https://doi.org/10.1371/journal.pone.0040357
Jhala G, Chee J, Trivedi PM et al (2016) Perinatal tolerance to proinsulin is sufficient to prevent autoimmune diabetes. JCI Insight 1(10):e86065. https://doi.org/10.1172/jci.insight.86065
Graham KL, Fynch S, Pappas EG, Tan C, Kay TWH, Thomas HE (2016) Isolation and culture of the islets of Langerhans from mouse pancreas. BioProtocol 6(12):e1840. https://doi.org/10.21769/BioProtoc.1840
Prantner D, Perkins DJ, Lai W et al (2012) 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J Biol Chem 287(47):39776–39788. https://doi.org/10.1074/jbc.M112.382986
Kallionpaa H, Elo LL, Laajala E et al (2014) Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes 63(7):2402–2414. https://doi.org/10.2337/db13-1775
Lundberg M, Krogvold L, Kuric E, Dahl-Jorgensen K, Skog O (2016) Expression of Interferon-Stimulated Genes in Insulitic Pancreatic Islets of Patients Recently Diagnosed With Type 1 Diabetes. Diabetes 65(10):3104–3110. https://doi.org/10.2337/db16-0616
Richardson SJ, Rodriguez-Calvo T, Gerling IC et al (2016) Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 59(11):2448–2458. https://doi.org/10.1007/s00125-016-4067-4
Thomas HE, Parker JL, Schreiber RD, Kay TW (1998) IFN-gamma action on pancreatic beta cells causes class I MHC upregulation but not diabetes. J Clin Invest 102(6):1249–1257. https://doi.org/10.1172/JCI2899
Chee J, Ko HJ, Skowera A et al (2014) Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol 192(2):572–580. https://doi.org/10.4049/jimmunol.1302100
Lieberman SM, Evans AM, Han B et al (2003) Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A 100(14):8384–8388. https://doi.org/10.1073/pnas.0932778100
Crow YJ, Manel N (2015) Aicardi-Goutieres syndrome and the type I interferonopathies. Nat Rev Immunol 15(7):429–440. https://doi.org/10.1038/nri3850
Shen N, Fu Q, Deng Y et al (2010) Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A 107(36):15838–15843. https://doi.org/10.1073/pnas.1001337107
Gateva V, Sandling JK, Hom G et al (2009) A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41(11):1228–1233. https://doi.org/10.1038/ng.468
Lee-Kirsch MA, Gong M, Chowdhury D et al (2007) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet 39(9):1065–1067. https://doi.org/10.1038/ng2091
Yasutomo K, Horiuchi T, Kagami S et al (2001) Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet 28(4):313–314. https://doi.org/10.1038/91070
Al-Mayouf SM, Sunker A, Abdwani R et al (2011) Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet 43(12):1186–1188. https://doi.org/10.1038/ng.975
Gunther C, Kind B, Reijns MA et al (2015) Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Invest 125(1):413–424. https://doi.org/10.1172/JCI78001
Smyth DJ, Cooper JD, Bailey R et al (2006) A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet 38(6):617–619. https://doi.org/10.1038/ng1800
Lincez PJ, Shanina I, Horwitz MS (2015) Reduced expression of the MDA5 Gene IFIH1 prevents autoimmune diabetes. Diabetes 64(6):2184–2193. https://doi.org/10.2337/db14-1223
Tai N, Wong FS, Wen L (2013) TLR9 deficiency promotes CD73 expression in T cells and diabetes protection in nonobese diabetic mice. J Immunol 191(6):2926–2937. https://doi.org/10.4049/jimmunol.1300547
Carrero JA, Benshoff ND, Nalley K, Unanue ER (2018) Type I and II Interferon Receptors Differentially Regulate Type 1 Diabetes Susceptibility in Male Versus Female NOD Mice. Diabetes 67(9):1830–1835. https://doi.org/10.2337/db18-0331
Schoggins JW, MacDuff DA, Imanaka N et al (2014) Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505(7485):691–695. https://doi.org/10.1038/nature12862
Sharma S, Campbell AM, Chan J et al (2015) Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci U S A 112(7):E710–E717. https://doi.org/10.1073/pnas.1420217112
Lemos H, Mohamed E, Huang L et al (2019) Stimulator of interferon genes agonists attenuate type I diabetes progression in NOD mice. Immunology 158(4):353–361. https://doi.org/10.1111/imm.13122
Walker MM, Crute BW, Cambier JC, Getahun A (2018) B Cell-Intrinsic STING Signaling Triggers Cell Activation, Synergizes with B Cell Receptor Signals, and Promotes Antibody Responses. J Immunol 201(9):2641–2653. https://doi.org/10.4049/jimmunol.1701405
Motwani M, Pesiridis S, Fitzgerald KA (2019) DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet 20(11):657–674. https://doi.org/10.1038/s41576-019-0151-1
Sivick KE, Desbien AL, Glickman LH et al (2018) Magnitude of Therapeutic STING Activation Determines CD8(+) T Cell-Mediated Anti-tumor Immunity. Cell Rep 25(11):3074–3085 e3075. https://doi.org/10.1016/j.celrep.2018.11.047
Acknowledgements
We thank C. Tan, C. Anthony, V. Moshovakis, V. Madafferi, H. Barlow and A. Cornelisz (St Vincent’s Institute) for technical support, genotyping and animal husbandry. CRISPR/Cas9 gene editing in NOD mice was performed by L. Hawkey, D. Truman and I. Smyth (Australian Phenomics Facility, Monash University, Clayton, Australia). Some of the data were presented as an abstract at The Australasian Society for Immunology 2017 Annual Scientific Meeting.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This work was funded by National Health and Medical Research Council of Australia (NHMRC) Program grants (GNT1126237 and GNT1150425), a JDRF Innovation Award (1-INO-2016-218-A-N) and fellowships from the JDRF (3-PDF-2017-379-A-N) and Manpei Suzuki Diabetes Foundation (SA). The St Vincent’s Institute receives support from the Operational Infrastructure Support Scheme of the Government of Victoria.
Author information
Authors and Affiliations
Contributions
SA, LM, GJ, SF, TC, CS, KLG, CJK and EGP acquired data, analysed and interpreted data and critically revised the manuscript. SA, APRS, BK, TWHK, TCB and HET made substantial contribution to conception and design of the study and critically drafted and revised the article for important intellectual content. All authors approved the final version of the manuscript. HET is responsible for the integrity of the work as a whole.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 360 kb)
Rights and permissions
About this article
Cite this article
Akazawa, S., Mackin, L., Jhala, G. et al. Deficiency of the innate immune adaptor STING promotes autoreactive T cell expansion in NOD mice. Diabetologia 64, 878–889 (2021). https://doi.org/10.1007/s00125-020-05378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05378-z