Abstract
Aims/hypothesis
Renal GLUT2 is increased in diabetes, thereby enhancing glucose reabsorption and worsening hyperglycaemia. Here, we determined whether loss of Glut2 (also known as Slc2a2) specifically in the kidneys would reverse hyperglycaemia and normalise body weight in mouse models of diabetes and obesity.
Methods
We used the tamoxifen-inducible CreERT2-Lox system in mice to knockout Glut2 specifically in the kidneys (Ks-Glut2 KO) to establish the contribution of renal GLUT2 to systemic glucose homeostasis in health and in insulin-dependent as well as non-insulin-dependent diabetes. We measured circulating glucose and insulin levels in response to OGTT or IVGTT under different experimental conditions in the Ks-Glut2 KO and their control mice. Moreover, we quantified urine glucose levels to explain the phenotype of the mice independently of insulin actions. We also used a transcription factor array to identify mechanisms underlying the crosstalk between renal GLUT2 and sodium–glucose cotransporter 2 (SGLT2).
Results
The Ks-Glut2 KO mice exhibited improved glucose tolerance and massive glucosuria. Interestingly, this improvement in blood glucose control was eliminated when we knocked out Glut2 in the liver in addition to the kidneys, suggesting that the improvement is attributable to the lack of renal GLUT2. Remarkably, induction of renal Glut2 deficiency reversed hyperglycaemia and normalised body weight in mouse models of diabetes and obesity. Longitudinal monitoring of renal glucose transporters revealed that Sglt2 (also known as Slc5a2) expression was almost abolished 3 weeks after inducing renal Glut2 deficiency. To identify a molecular basis for this crosstalk, we screened for renal transcription factors that were downregulated in the Ks-Glut2 KO mice. Hnf1α (also known as Hnf1a) was among the genes most downregulated and its recovery restored Sglt2 expression in primary renal proximal tubular cells isolated from the Ks-Glut2 KO mice.
Conclusions/interpretation
Altogether, these results demonstrate a novel crosstalk between renal GLUT2 and SGLT2 in regulating systemic glucose homeostasis via glucose reabsorption. Our findings also indicate that inhibiting renal GLUT2 is a potential therapy for diabetes and obesity.
Graphical abstract

Similar content being viewed by others

Introduction
GLUT2 is a major facilitative glucose transporter expressed in the basolateral membrane of the epithelial cells predominantly in the liver, kidneys and intestine [1, 2]. It is also present in rodent beta cells [3, 4]; however, some reports suggest that GLUT2 is not a major glucose transporter in human beta cells [4, 5]. GLUT2 (also known as SLC2A2) mutations cause Fanconi–Bickel syndrome [6, 7], which is characterised by glucose malabsorption, glycogen accumulation and renal glucosuria. Although this syndrome does not cause fasting hyperglycaemia [6, 7], it may lead to transient neonatal diabetes [8, 9]. This phenotype is in contrast to that observed in global Glut2 knockout (KO) mice, which exhibit overt diabetes [3, 10]. Renal GLUT2 facilitates most of the glucose reabsorption in concert with sodium–glucose cotransporter 2 (SGLT2), which is located at the apical membrane of renal proximal tubular cells and is an established target in the treatment of diabetes [11,12,13,14,15,16,17,18]. Diabetes increases renal GLUT2 levels in humans and rodents [19,20,21,22,23,24,25], which further worsens hyperglycaemia because of enhanced glucose reabsorption. Therefore, blocking renal GLUT2 may ameliorate hyperglycaemia by interfering with this vicious cycle. Yet, it is unknown whether loss of function of renal GLUT2 would adequately elevate glucosuria to reverse hyperglycaemia to the degree that is accomplished by SGLT2 inhibition [13,14,15,16,17,18]. Moreover, the physiological contribution of renal GLUT2 to systemic glucose homeostasis is not completely established.
Previous studies, including those from our laboratory, suggest that reducing renal GLUT2 may mitigate hyperglycaemia [24, 26,27,28]. For example, we recently reported that reduction of GLUT2 levels in the kidneys improves glucose tolerance in hypothalamus-specific pro-opiomelanocortin (POMC) or melanocortin 4 receptor (MC4R) KO mice despite obesity and insulin resistance [27, 28]. Nevertheless, whether lack of renal GLUT2 would reverse hyperglycaemia in mouse models of diabetes and obesity remains unknown. To answer this question, we generated mice in which renal Glut2 can be knocked out at a desired time using the tamoxifen-inducible CreERT2-Lox system. Moreover, we produced and characterised a mouse model that lacks Glut2 in the liver in addition to the kidneys to further validate the precise contribution of renal GLUT2 in regulating blood glucose relative to other major tissues expressing this glucose transporter. Besides establishing the physiological role of renal GLUT2 in systemic glucose homeostasis and its therapeutic potential in diabetes and obesity in this study, we investigated whether and how the loss of renal Glut2 affects the gene expression of other renal glucose transporters.
Methods
Study design and mouse models
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Rochester, Pennington Biomedical Research Center, or the University of Alabama at Birmingham, and were performed according to the US Public Health Service guidelines for the humane care and use of experimental animals. Mice were housed in ventilated cages under controlled temperature (~23°C) and photoperiod (12 h light–dark cycle, lights on from 06:00 hours to 18:00 hours) conditions with free access to Hydropac water (Lab Products, USA) and regular laboratory chow (5010; LabDiet, USA). After genotyping, mice were randomly assigned to different experimental groups. We used two mouse lines with their littermate controls in this study: KspCadCreERT2;Glut2loxP/loxP mice with induced renal Glut2 deficiency; and Ggt1Cre;Glut2loxP/loxP mice with knockout of Glut2 in the liver in addition to the kidneys.
We generated Glut2loxP/loxP (also known as Slc2a2loxP/loxP) mice (received from D. McDougal, Pennington Biomedical Research Center, Baton Rouge, LA, USA) using heterozygous Glut2tm1a(KOMP)Wtsi mice that were produced using ES cells obtained from the Knockout Mouse Project at the University of California, Davis (project ID: CSD40514; MMRRC:065826-UCD). ES cells were microinjected into albino C57BL/6J mouse blastocysts and the resulting chimeras were mated with B6(Cg)-Tyrc-2J/J (B6-albino mice, 000058; The Jackson Laboratory, USA) for germline-derived F1 mice. F1 mice were mated with C57BL/6NTac mice for colony expansion. These founder mice were crossed with B6.Cg-Tg(ACTFLPe)9205Dym/J mice (005703; The Jackson Laboratory), which express the flippase recombinase to remove the lacZ and neomycin cassettes generating Glut2loxP/loxP mice (i.e. tm1a > tm1c allele conversion), which can be used for Cre-mediated Glut2 inactivation (ESM Fig. 1a). To generate KspCadCreERT2;Glut2loxP/loxP and their littermate control mice, we first crossed the Glut2loxP/loxP mice with the KspCadCreERT2 mouse line [29, 30] (received from D. J. M. Peters, Leiden University Medical Center, Leiden, the Netherlands), followed by a second generation of intercrossing between KspCadCreERT2;Glut2loxP/+ and Glut2loxP/loxP mice. The KspCadCreERT2 mice are validated to exhibit Cre expression in proximal tubules [29,30,31], wherein Glut2 is also expressed predominantly. We used the same breeding strategy to produce Ggt1Cre;Glut2loxP/loxP mice from the Ggt1Cre mouse line (012841; The Jackson Laboratory) resulting in Glut2 knockout in the liver in addition to the kidneys. All the experimental mice used in this study were age-matched relative to their corresponding control groups.
We injected tamoxifen (50 mg/kg dissolved first in one part 100% ethanol by incubating the mixture at 60°C for 20 min, followed by addition of nine parts of sesame oil, T5648 and S3547 [Sigma, USA]) once daily for three consecutive days in KspCadCreERT2;Glut2loxP/loxP mice to induce Glut2 deficiency in the kidneys. All of the experiments, except those using a diet-induced obesity mouse model or noted otherwise, were completed within 14 days of inducing the Glut2 deficiency. To produce the mouse model of type 1 diabetes, we administered freshly prepared streptozotocin (STZ; 80 mg/kg dissolved in citric acid–sodium citrate buffer, pH 4.5, i.p.; S0130; Sigma) once daily for five consecutive days. The mice exhibited hyperglycaemia (>14 mmol/l glucose) by 24 days after the first STZ injection. To induce obesity and insulin resistance in mice used in this study, immediately after weaning we fed them with high-fat + sucrose diet (HFSD; D12331; Research Diets, USA), or the regular laboratory chow to the control group, for 20 weeks and measured their metabolic variables. After the baseline measurements, we induced renal Glut2 deficiency and the mice continued to receive their corresponding diets for an additional 5 weeks. We measured body weight and food intake of these mice once a week for the duration of the study. Experimenters were not blinded to group assignment and outcome assessment. We did not exclude any data, samples or mice in reporting the results of this study.
Reverse transcription quantitative PCR
An E.Z.N.A kit (101319-260; Omega, USA) was used to extract total RNA from the renal cortex, liver or primary renal proximal tubular cells. We used 500 ng total RNA and random hexamer primers (1708891; iScript cDNA synthesis kit; BioRad, USA) to generate cDNA. Reverse transcription quantitative PCR (RT-qPCR) was performed using a StepOne Real Time PCR System (Applied Biosystems, USA) and SYBR green master mix (1725124; BioRad). We used the following primers: for Glut2 (Slc2a2), 5′-GAA GGA ACT CAG TAC AGC AGT G-3′ and 5′-TCA TCC ACA TTC AGT ACA GGA C-3′; for Sglt2 (Slc5a2), 5′-AGT GTC TGT GTA CAT CAG TGC-3′ and 5′-CAA GAT CTC GGT GGA TAT GTT CTC-3′; for Glut1 (Slc2a1), 5′-GTG GTG AGT GTG GAT G-3′ and 5′-AGT TCG GCT ATA ACA CTG GTG-3′; for Sglt1 (Slc5a1), 5′-CAA TCA GCA CGA GGA TGA ACA-3′ and 5′-GCT CCT TGA CCT CCA TCT TC-3′; for Hnf1α (Hnf1a), 5′-AGA GAC CTT GGT GGA GTG T-3′ and 5′-GGC AAA CCA GTT GTA GAC ACG C-3′; for G6pc, 5′-AGG TCG TGG CTG GAG TCT TGT C-3′ and 5′-GTA GCA GGT AGA ATC CAA GCG C-3′; for Pck1α, 5′-GGC GAT GAC ATT GCC TGG ATG A-3′ and 5′-TGT CTT CAC TGA GGT GCC AGG A-3′; and for Hprt, 5′-AAC AAA GTC TGG CCT GTA TCC-3′ and 5′-CCC CAA AAT GGT TAA GGT TGC-3′. All primers were used at a final concentration of 500 nmol/l. The relative quantity of each mRNA was calculated from standard curves and normalised to the internal control Hprt, and then normalised to the mean of corresponding controls.
Immunohistochemistry, western blotting, and proteomics
We embedded formalin (10% neutral buffered)-fixed kidneys in paraffin and cut 5 μm sections with a microtome. We mounted these sections onto slides for immunohistochemistry. We had deparaffinised and rehydrated the sections before incubating them in sodium citrate buffer (pH 6.0) at 95°C in a water bath for 20 min for antigen retrieval. After the antigen retrieval step, we rinsed the slides three times (10 min/wash) in tris-buffered saline with 0.1% Tween 20 detergent (TBST) buffer and blocked the sections in 10% normal goat serum for 2 h. We then incubated the sections overnight at 4°C with the following primary antibodies: rabbit anti-GLUT2 (600-401-GN3 [Rockland Immunochemicals, USA] or ab54460 [Abcam, USA]; 1:500 dilution in TBST); mouse anti-SGLT2 (sc-393350; Santa Cruz Biotechnology, USA; 1:100 dilution in TBST); or mouse anti-HNF1α (sc-393925; Santa Cruz Biotechnology; 1:100 dilution in TBST). Following the incubation period, the sections were washed three times (10 min/wash) in TBST and then incubated with either secondary goat-anti-rabbit antibody conjugated to Alexa Fluor 594 (ab150080; Abcam; 1:1000 dilution in TBST) or goat-anti-mouse antibody conjugated to Alex Fluro 647 (ab150115; Abcam; 1:1000 dilution in TBST) for 1 h at room temperature. The sections were washed again three times (10 min/wash) in TBST and incubated for 1 h with lotus tetragonolobus lectin (FL-1321-2; Vector Laboratories, USA; 1:200 dilution in TBST) to label renal proximal tubules. After this incubation, the sections were washed with TBST (10 min, one wash) and counterstained with DAPI (D9542; Sigma; 1 μg/ml) for 1 min. The sections were washed again with TBST, then the slides were air-dried and coverslipped using ProLong Antifade mounting medium (P36930; Molecular Probes, USA). Images were captured using Keyence fluorescence microscope BZ-X800. We quantified the images using the NIH ImageJ software, version 1.53n (https://imagej.nih.gov/ij/download.html).
We performed western blotting to measure renal GLUT2 and hepatocyte nuclear factor-1α (HNF1α) as described previously [27, 28, 32] using these primary and secondary antibodies: anti-GLUT2 (600-401-GN3; Rockland Immunochemicals, USA; 1:2000 dilution in TBST), anti-HNF1α (89670; Cell Signaling Technology, USA; 1:2000 dilution in TBST) and anti-rabbit secondary antibody (NA934; GE Healthcare, USA; 1:5000 dilution in TBST). We had either cut the blots horizontally just above ~80 KDa to examine the internal control at the same time as the proteins of interest on the same membrane or we had stripped the blots to remove antibodies after imaging proteins of interest and used the same blots for probing the internal control. We used ECL substrate (34095; Thermo Fisher Scientific, USA) to produce luminescence that was recorded on a BioRad imaging system.
We used proteomics assay to validate the results obtained from the antibodies mentioned above and to further confirm the deficiency of renal GLUT2 protein in our new mouse model. Renal cortical trypsin-digested peptides, obtained from control and experimental mice, were injected onto a homemade 30 cm C18 column with 1.8 µm beads (Sepax, USA), with an Easy nLC-1200 HPLC (Thermo Fisher Scientific), connected to a Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific). Raw data was searched using the SEQUEST search engine within the Proteome Discoverer software platform, version 2.4 (Thermo Fisher Scientific), using the SwissProt mus musculus database. The Minora node was used to determine relative protein abundance between the samples using the Summed Abundance default settings. Percolator was used as the false discovery rate (FDR) calculator, filtering out peptides which had a q value greater than 0.01.
Primary mouse renal proximal tubular epithelial cells
We isolated primary proximal tubular epithelial cells from mice with Glut2 knocked out specifically in the kidneys (Ks-Glut2 KO mice) and their littermate controls using a published protocol [33]. We cultured the cells in collagen-coated six-well plates (106 cells/well; A1142801; Thermo Fisher Scientific, USA) in renal epithelial cell basal medium (PCS-400-030; ATCC, USA) supplemented with the renal epithelial cell growth kit (PCS-400-040; ATCC). Two days after initiating the culture of these cells, we used them for either gene expression measurements or glucose transport assay.
For gene expression studies, we transfected the cells with Hnf1α-expressing plasmid (MC202766; OriGene Technologies, USA), control plasmid (PCMV6KN; OriGene Technologies) or Glut2-expressing plasmid (MG208388; OriGene Technologies) using TurboFectin transfection reagent (TF81001; OriGene Technologies) according to the manufacturer’s protocol. Three days after the transfection, we extracted the RNA and used RT-qPCR to measure the mRNA levels of Glut2, Sglt2 and Hnf1α.
Glucose transport assay
We measured renal GLUT2 activity using a glucose transport assay. For this assay, we cultured the primary mouse renal proximal tubular epithelial cells in hanging inserts (MCRP24H48; Millipore, USA) as previously described [28]. We incubated the cells with Hank’s balanced salt solution containing [13C6]glucose (5 mmol/l) in the presence or absence of the GLUT2 inhibitor phloretin (1 mmol/l in 1:1 DMSO/PBS; 14452; Cayman Chemical, USA). We then measured the labelled glucose at the basolateral (bottom) side of the cells at different times. The University of Rochester Mass Spectrometry Resource Laboratory measured 13C6 glucose by LC-MS assay using a Shonex HILICpak VG-50 2D column.
OGTT, ITT and in vivo glucose-stimulated insulin secretion assay
We performed OGTTs in mice fasted overnight (18:00–08:00 hours) or for 6 h (08:00–14:00 hours). For ITTs we fasted mice for 6 h (08:00–14:00 hours). On the day of the experiments we measured their baseline blood glucose levels (tail-vein blood; AlphaTRAK 2 glucometer; Zoetis, USA) at 0 min and administered glucose by oral gavage (100 mg/mouse in 300 μl 0.9% wt/vol. saline [154 mmol/l NaCl]; G8270; Sigma) or insulin by i.p. injection (15 mU/mouse; Humulin R; Eli Lilly, USA), followed by measurements of blood glucose levels at 15, 30, 60 and 120 min. In mice with STZ-induced diabetes, we administered only 15 mg glucose/mouse to assess glucose tolerance because a higher dose would have increased blood glucose levels beyond the sensitivity of the assay range due to this model’s hyperglycaemia at baseline. To calculate the total AUC for the glucose measurements, we used the trapezoidal rule.
To assess glucose-stimulated insulin secretion (GSIS) in KspCadCreERT2;Glut2loxP/loxP mice and their littermate controls, we cannulated the carotid artery and jugular vein to administer glucose and collect blood in freely moving awake mice [27]. After the mice recovered from the surgery over a period of 3–5 days, on the day of the experiment, we fasted mice for 6 h (08:00–14:00 hours) and administered an i.v. bolus of 60 mg of glucose. After the glucose administration we measured blood glucose and insulin levels at different times. For GSIS assay in Ggt1Cre;Glut2loxP/loxP mice and their control littermates, we fasted mice for 6 h and collected tail blood using capillary tubes (22-362-566; Fisherbrand, USA) at baseline and at 20 min after oral challenge with 100 mg glucose.
For glucose tolerance studies involving the SGLT2 inhibitor dapagliflozin, we administered the drug by i.p. injection (5 mg/kg dissolved in 300 μl 0.9% wt/vol. saline; 11574; Cayman Chemical) 30 min prior to the GTTs.
Urine glucose, sodium and creatinine assays
We placed mice individually in metabolic cages (Tecniplast, USA) and allowed them to acclimate to the cages for 3–5 days before collecting their 24 h urine under different experimental conditions. We used colorimetric assays to measure glucose (10009582; Cayman Chemical), sodium (MAK247; Sigma) and creatinine (500701; Cayman Chemical) levels in the urine according to the manufacturers’ protocols.
For urine glucose studies involving dapagliflozin, we administered the drug by i.p. injection (5 mg/kg dissolved in 300 μl 0.9% wt/vol. saline) twice a day (09:00 and 17:00 hours) and collected urine 24 h after the first injection.
Plasma or serum measurements
We collected the mouse trunk blood between 14:00 and 15:00 hours on the day of the experiments to obtain serum from mice in all the experimental groups. For plasma, we used heparinised capillary tubes (22-362-566; Fisherbrand) to draw tail-vein blood from the mice used in this study. We centrifuged the blood at 2000 g at 4°C for 20 min to separate plasma or serum from whole blood. We measured plasma insulin (90080; Crystal Chem, USA) and serum glucagon (81518; Crystal Chem) levels by ELISA. Serum creatinine (700460; Cayman Chemical), β-hydroxybutyrate (700190; Cayman Chemical) and NEFA (ab65341; Abcam) were measured by colorimetric assays. The absorbance was measured using a spectrophotometer as per the manufacturer’s instructions (Epoch, BioTek, USA).
GFR, glomerular area and urine pH
GFR in conscious mice was measured using a transdermal FITC–sinistrin clearance method [34] at the University of Alabama at Birmingham O’Brien Center. Mice were anaesthetised with isoflurane and FITC–sinistrin (MediBeacon, USA) solution injected via the tail vein. Data were analysed using MB Studio version 2.1 (MediBeacon). A three-compartment model with linear baseline correction term (CP3L) was used to provide best fit results, as relating to t½ measurements. The FITC–sinistrin t½ (in min) was converted to GFR (in μl/min) and normalised for individual mouse body weight.
For measuring the glomerular area, we used 5 μm kidney sections obtained from formalin-fixed paraffin embedded mouse kidneys. The sections were stained with H&E. Images of the sections were captured using an Olympus microscope with ×20 objective lens and analysed using the NIH ImageJ software version 1.53n, https://imagej.nih.gov/ij/download.html.
We measured urine pH using an electronic high resolution pH meter (HI2020-01; Hanna Instruments, USA).
PCR array
For PCR array, the cDNA was mixed with nuclease-free water and SYBR Green master mix to prepare PCR components mix. We added 25 μl of the components mix to each well of the mouse transcription factors PCR array plate (PAMM-075ZC; Qiagen, USA) and performed RT-qPCR as described above. The relative gene expression was calculated using the \( {2}^{-\Delta \Delta {\mathrm{C}}_{\mathrm{t}}} \) method as per the manufacturer’s instructions. We used β-actin as an internal control for the quantification of target genes.
Statistical analyses
All data are presented as mean ± SEM and were analysed by two-tailed Student’s paired or unpaired t test, one-way ANOVA or two-way ANOVA followed by a Bonferroni post hoc multiple comparison test when appropriate. All analyses were performed using Prism version 8.0.1 (GraphPad, USA) and differences were considered statistically significant at p<0.05.
Results
Generation and validation of mice showing inducible kidney-specific loss of Glut2
We crossed the mouse line expressing tamoxifen-inducible Cre recombinase under the control of kidney-specific cadherin gene (KspCadCreERT2) [29] with Glut2loxP/loxP mice (ESM Fig. 1a) to obtain KspCadCreERT2;Glut2loxP/loxP mice. The KspCadCreERT2 mouse line is well characterised for inducing gene modifications specific to the kidney, including proximal tubules where Glut2 is predominantly present, using the Cre-Lox system [29,30,31]. We observed that the KspCadCreERT2;Glut2loxP/loxP mice exhibited deficiency of renal Glut2 gene and GLUT2 protein as well as reduced glucose transport activity compared with their littermate controls (KspCadCreERT2 or Glut2loxP/loxP) following the administration of tamoxifen, without Glut2 expression in the liver being affected (male mice, Fig. 1a–d and ESM Fig. 1b,c; female mice, ESM Fig. 2a). Moreover, the expression of other renal cortical glucose transporters in the experimental group was not different from that in the control group within 14 days of inducing the Glut2 deficiency (male mice, Fig. 1c,e–h; female mice, ESM Fig. 2b–d). Overall, we have validated tamoxifen-inducible lack of Glut2 specifically in the kidneys (Ks-Glut2 KO) in the new mouse model.
Validation of inducible kidney-specific loss of Glut2 in mice. (a) Representative genotypes of mice used in this study. Glut2loxP mouse model: Glut2loxP heterozygous (705 and 566 bp); Glut2loxP homozygous (705 bp). KspCadCreERT2 mouse model: KspCadCreERT2 heterozygous (700 bp); WT (no band). (b–e) Expression of renal cortical and hepatic Glut2 (b), and immunofluorescence staining of renal GLUT2 and SGLT2 (c), together with their quantification (d, e). Scale bar, 100 μm. (f–h) Expression of renal Sglt2 (f), Glut1 (g) and Sglt1 (h). Data are shown as means ± SEM for 8- to 12-week-old male mice within 14 days of inducing renal Glut2 deficiency, n = 5–7. ***p<0.001 vs Ctrl group (one-way ANOVA followed by Bonferroni’s post hoc test or two-tailed unpaired Student’s t test). Ctrl, control group; Het, heterozygous; Hom, homozygous; LTL, Lotus tetragonolobus lectin, a marker of renal proximal tubules; NC, negative control; WT, wild-type
Ks-Glut2 KO mice exhibit improved glucose tolerance and intense glucosuria
The Ks-Glut2 KO mice had normal body weight, fasting blood glucose levels, and improved glucose tolerance (male mice, Fig. 2a–c; female mice, ESM Fig. 2e,f) without any changes in GSIS or insulin sensitivity (male mice, Fig. 2d,e; female mice, ESM Fig. 2g). The KO mice manifested polyuria and massive glucosuria (male mice, Fig. 2f–h; female mice, ESM Fig. 2h–j). Urine glucose concentration (Fig. 2f and ESM Fig. 2h) and urine volume (Fig. 2g and ESM Fig. 2i) were used to calculate the total amount of glucose excreted in 24 h (Fig. 2h and ESM Fig. 2j). Moreover, the Ks-Glut2 KO mice exhibited polydipsia, natriuresis and reduced amount of urine creatinine compared with their littermate controls (ESM Fig. 3a–c) without any changes in food intake (control mice, 4.1 ± 0.3 g/day; Ks-Glut2 KO mice, 3.9 ± 0.2 g/day). Together, these results demonstrate the physiological contribution of renal GLUT2 in regulating systemic glucose homeostasis by glucosuria.
Lack of renal Glut2 improves glucose tolerance and elevates glucosuria in mice. (a) Body weight. (b) Blood glucose levels during OGTT, with corresponding AUC. (c) Blood glucose levels during IVGTT. (d) Plasma insulin levels during GSIS. (e) Blood glucose levels during ITT, with corresponding AUC. (f) 24 h urine glucose concentration. (g) 24 h urine volume. (h) 24 h amount of glucose in urine. Data are shown as means ± SEM for 8- to 12-week-old male mice within 14 days of inducing renal Glut2 deficiency, n = 5–7. **p<0.01 and ***p<0.001 vs Ctrl group (two-tailed unpaired Student’s t test or repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test). Ctrl, control group
The Ks-Glut2 KO mice had normal serum glucagon, β-hydroxybutyrate, NEFA and creatinine levels (ESM Fig. 3d–g). Moreover, of the two major enzymes involved in hepatic gluconeogenesis (Pck1 and G6pc), only G6pc mRNA was elevated in the Ks-Glut2 KO mice (ESM Fig. 4a,b), indicating that increased gluconeogenesis by G6pc was likely to be one of the sources of endogenous glucose that makes up for the loss of glucose in urine. The KO mice had normal GFR, glomerular area and urine pH (ESM Fig. 4c–i), as well as normal kidney weights (both the kidneys combined: control mice, 12 ± 0.2 mg/g body weight; KO mice, 12.8 ± 0.2 mg/g body weight), suggesting the absence of any general renal dysfunction or pathology in these mice.
Mice lacking Glut2 in the liver in addition to the kidneys do not show improved glucose tolerance despite elevated glucosuria
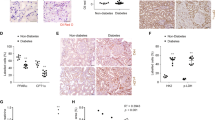
To define and validate the precise contribution of renal GLUT2 to glucose homeostasis relative to the role of other major tissues that also express this glucose transporter, we generated a mouse model that exhibited Glut2 deficiency in the kidneys and the liver simultaneously by breeding Ggt1Cre with the Glut2loxP/loxP mice. We had validated the loss of Glut2 in the liver and renal cortex in Ggt1Cre;Glut2loxP/loxP mice (Fig. 3a,b).
Mice lacking Glut2 in the liver in addition to the kidneys show impaired glucose tolerance despite elevated glucosuria. (a) Representative genotypes of mice used in this study. Glut2loxP mouse model: Glut2loxP heterozygous (705 and 566 bp); and Glut2loxP homozygous (705 bp). Ggt1Cre mouse model: Ggt1Cre homozygous (100 bp); and WT (no band). (b) Renal cortical and hepatic Glut2 expression. (c) Blood glucose levels during OGTT, with corresponding AUC. (d) 24 h urine glucose concentration. (e) 24 h urine volume. (f) 24 h amount of glucose in urine. (g) Plasma insulin levels during oral glucose challenge. (h) Serum glucagon levels. Data are shown as means ± SEM for 8- to 12-week-old male mice, n = 4–6. *p<0.05 and ***p<0.001 vs Ctrl group (two-tailed unpaired Student’s t test, or one-way ANOVA or repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test). Ctrl, control group; Het, heterozygous; Hom, homozygous; K+L Glut2 KO, mice with Glut2 deficiency in the kidneys and liver; NC, negative control; PC, positive control; WT, wild-type
The mice with Glut2 deficiency in the kidneys and liver (K+L Glut2 KO) showed impaired glucose tolerance despite massive glucosuria and associated polyuria (male mice, Fig. 3c–f; female mice, ESM Fig. 5a–d). Reduced GSIS (male mice, Fig. 3g; female mice, ESM Fig. 5e) in the K+L Glut2 KO mice may explain the impairment in their glucose tolerance. Moreover, the K+L Glut2 KO mice had higher serum glucagon levels (male mice, Fig. 3h; female mice, ESM Fig. 5f), which may have further contributed to their impaired glucose tolerance. These findings reveal the differential physiological contribution of tissue-specific GLUT2 in systemic glucose homeostasis.
Lack of renal Glut2 reverses hyperglycaemia, improves glucose tolerance and normalises body weight in mouse models of diabetes and obesity
In a longitudinal study using mouse models of STZ-induced type 1 diabetes, we observed that Ks-Glut2 KO mice recovered from STZ-induced hyperglycaemia independently of changes in body weight (Fig. 4a,b) and displayed more severe glucosuria and polyuria (Fig. 4c–e) compared with their control littermates. Notably, the control mice with STZ-induced hyperglycaemia displayed increased renal Glut2 expression (Fig. 4f) but reduced Sglt2 mRNA levels (Fig. 4g) compared with the mice that did not receive STZ. These results, which agree with findings from previous studies [19,20,21,22,23,24, 35], suggest that the reduced renal Sglt2 could be a compensatory response to suppress glucose reabsorption in diabetes. Despite this decrease in renal Sglt2, it was only after induction of renal Glut2 deficiency that the STZ-induced hyperglycaemia was reversed.
Renal Glut2 deficiency reverses hyperglycaemia and normalises body weight in mouse models of diabetes and obesity. (a) Fasting blood glucose levels at baseline, after inducing type 1 diabetes by STZ and on the seventh day after knocking out renal Glut2 by tamoxifen. (b–e) Body weight (b), 24 h urine glucose concentration (c), 24 h urine volume (d) and 24 h amount of glucose in urine (e) in 8- to 12-week-old female mice with STZ-induced type 1 diabetes before, and on the 12th day after, knocking out renal Glut2 using tamoxifen. (f, g) Expression of renal Glut2 (f) and Sglt2 (g) in female mice with STZ-induced type 1 diabetes before, and on the 14th day after, knocking out renal Glut2. (h) Changes in body weight during 25 weeks of regular diet or HFSD feeding. (i) Changes in the weight of epididymal adipose tissue 4 weeks after inducing renal Glut2 deficiency. (j, k) Blood glucose levels during OGTT at 1 week pre-renal Glut2 deficiency (j) and on the fifth day post-renal Glut2 deficiency (k). (l) Plasma insulin levels 3 days before and on eighth day after renal Glut2 deficiency. (m–o) 24 h urine glucose concentration (m), 24 h urine volume (n) and 24 h amount of glucose in urine (o) on the tenth day after inducing renal Glut2 deficiency in male mice fed a regular diet or HFSD. Data are shown as means ± SEM, n = 5. *p<0.05 and ***p<0.001 vs baseline (a) or Ctrl + Sal (f, g); †p<0.05 and †††p<0.001 vs STZ (a) or Ctrl + STZ (c–f); ‡p<0.05, ‡‡p<0.01 and ‡‡‡p<0.001 vs Ctrl + RD; §p<0.05, §§p<0.01 and §§§p<0.001 vs Ctrl + HFSD; (two-tailed unpaired Student’s t test, or one-way ANOVA or repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test). Ctrl, control Glut2loxP/loxP mice; RD, regular diet; TAM, tamoxifen
In the HFSD-induced obesity model, we observed that renal Glut2 deficiency normalised body weight (Fig. 4h), reduced the weight of epididymal fat (Fig. 4i), restored fasting blood glucose and plasma insulin levels, and improved glucose tolerance (Fig. 4j-l) likely via elevated glucosuria (Fig. 4m–o). The renal Glut2 deficiency did not change food intake (control mice fed HFSD, 2.4 ± 0.02 g/day; Ks-Glut2 KO mice fed HFSD, 2.3 ± 0.1 g/day), indicating that the reversal of obesity was not due to decreased food consumption. Like STZ, HFSD also increased renal Glut2 (control diet, 100 ± 15%; HFSD, 141 ± 9% of the control; p<0.05) and reduced Sglt2 (control diet, 100 ± 6%; HFSD, 72 ± 8% of the control; p<0.05) mRNA levels, again indicating the importance of inhibiting renal GLUT2 to counteract the enhanced glucose reabsorption caused by diabetes or obesity. At the end of the study, we did verify tamoxifen-induced loss of renal Glut2 (control mice fed HFSD, 100 ± 14%; Ks-Glut2 KO mice fed HFSD, 7 ± 1% of the control; p<0.001) in HFSD-fed KspCadCreERT2;Glut2loxP/loxP mice. Collectively, these findings justify the need for blocking renal GLUT2 for the treatment of diabetes and obesity.
SGLT2 inhibition further improves blood glucose control in Ks-Glut2 KO mice
Dapagliflozin (5 mg/kg, administered by i.p. injection 30 min prior to OGTT) further improved glucose clearance and enhanced glucosuria in the Ks-Glut2 KO mice (Fig. 5a–d). Based on these promising results, we also evaluated the effects of SGLT2 inhibition on glucose tolerance in Ks-Glut2 KO mice with either STZ-induced insulin deficiency or HFSD-mediated insulin resistance as described in the longitudinal study mentioned above. Interestingly, dapagliflozin further improved glucose tolerance in these insulin-deficient (Fig. 5e) or insulin-resistant Ks-Glut2 KO mice (Fig. 5f). Moreover, dapagliflozin (5 mg/kg by i.p. injection at 09:00 and 17:00 hours) increased polyuria, which elevated the total amount of glucose excreted in urine in the Ks-Glut2 KO mice (Fig. 5g–i). These findings suggest that combined inhibition of SGLT2 and renal GLUT2 may achieve optimal blood glucose control in diabetes.
Dapagliflozin improves glucose tolerance in the absence and presence of diabetes in mice lacking renal Glut2. (a) Blood glucose levels during OGTT, with corresponding AUC, in 8- to 12-week-old female mice on the seventh day after inducing renal Glut2 deficiency. (b–d) 24 h urine glucose concentration (b), 24 h urine volume (c) and 24 h amount of glucose in urine (d) on the 12th day after inducing renal Glut2 deficiency in 8- to 12-week-old female mice. (e) Blood glucose levels during OGTT, with corresponding AUC, on the eighth day after inducing renal Glut2 deficiency in 8-week-old female mice with STZ-induced type 1 diabetes. (f–i) Blood glucose levels during OGTT, with corresponding AUC (f), 24 h urine glucose concentration (g), 24 h urine volume (h) and 24 h amount of glucose in urine (i) on the 14th day after inducing renal Glut2 deficiency in HFSD-fed mice. Data are shown as means ± SEM, n = 5 or 6. *p<0.05, **p<0.01 and ***p<0.001 vs Ctrl + Sal; †p<0.05, ††p<0.01 and †††p<0.001 vs Ctrl + DAPA; ‡p<0.05, ‡‡p<0.01 and ‡‡‡p<0.001 vs Ks-Glut2 KO + Sal; §p<0.05, §§p<0.01 and §§§p<0.001 vs Ctrl + HFSD + Sal; ¶p<0.05 and ¶¶p<0.01 vs Ctrl + HFSD + DAPA; ¥p<0.05 and ¥¥ p<0.01 vs Ks-Glut2 KO + HFSD +Sal; (repeated measures two-way ANOVA followed by Bonferroni’s multiple comparison test). Ctrl, control group; DAPA, dapagliflozin; Sal, saline
Long-term renal Glut2 deficiency almost eliminates Sglt2 expression via downregulating hepatocyte nuclear factor 1-α
To determine the long-term effects of renal Glut2 deficiency on other renal glucose transporters, we measured their expressions 3 weeks after knocking out renal Glut2. Unexpectedly, this long-term renal Glut2 deficiency dramatically reduced Sglt2 gene and SGLT2 protein levels (male mice, Fig. 6a–d; female mice, ESM Fig. 6a,b); Sglt1 was decreased by about 20% without Glut1 levels being affected (male mice, Fig. 6e,f; female mice, ESM Fig. 6c,d). We had also verified these striking findings using proteomics (ESM Table 1), which showed that there was about 70–80% decrease in renal cortical GLUT2 and SGLT2 as well as no change in GLUT1 and sodium–glucose cotransporter 1 (SGLT1) levels. Therefore, the Ks-Glut2 KO mice could be utilised as a Ks-Glut2 and -Sglt2 double KO mouse model 3 weeks after inducing renal Glut2 deficiency.
Long-term renal Glut2 deficiency almost abolishes the expression of Sglt2 by downregulating Hnf1α. (a, b) Expression of renal cortical Glut2 (a) and Sglt2 (b). (c, d) Immunofluorescence staining of renal SGLT2, with its quantification. Scale bar, 100 μm. (e–i) Expression of renal cortical Glut1 (e), Sglt1 (f) and Hnf1α (g), and immunofluorescence staining of renal HNF1α as well as its quantification (h, i), on the 21st day following renal Glut2 deficiency in 8- to 12-week-old male mice. Scale bar, 100 μm. (j) mRNA levels of Hnf1α, Sglt2 and Glut2 in primary renal proximal tubular epithelial cells isolated from 8- to 12-week-old male control (Glut2loxP/loxP) mice, or mice with kidney-specific loss of Glut2 (Ks-Glut2 KO) on the 21st day following renal Glut2 deficiency. Data are shown as means ± SEM, n = 5 or 6. ***p<0.001 vs Ctrl; ‡‡‡p<0.001 vs Ks-Glut2 KO + Glut2; †††p<0.001 vs Ks-Glut2 KO + control plasmid group (two-tailed unpaired Student’s t test or two-way ANOVA followed by Bonferroni’s multiple comparison test). Ctrl, control Glut2loxP/loxP group; HNF1α, hepatocyte nuclear factor-1α; Ks-Glut2 KO, Glut2 knocked out specifically in the kidneys; Ks-Glut2 KO + Hnf1α or Glut2, primary renal proximal tubular epithelial cells isolated from Ks-Glut2 KO mice and transfected with Hnf1α or Glut2 expressing plasmids; LTL, Lotus tetragonolobus lectin, a marker of renal proximal tubules
To identify the molecular mechanisms underlying the crosstalk between renal Glut2 and Sglt2, we used a PCR array to screen for renal mouse transcription factors that are downregulated in Ks-Glut2 KO mice. We observed that a few genes were downregulated by 40–50% (ESM Table 2). Among these genes, Hnf1α seemed a likely mediator of the crosstalk between renal GLUT2 and SGLT2 because of its known role in controlling Sglt2 expression [36,37,38]. We validated the downregulation of renal Hnf1α gene and protein in Ks-Glut2 KO mice using RT-qPCR, immunohistochemistry and western blotting (male mice, Fig. 6g–i and ESM Fig. 6e; female mice, ESM Fig. 6f). Notably, short-term (within 14 days) renal Glut2 deficiency did not affect renal Hnf1α mRNA levels (control mice, 100 ± 18%; Ks-Glut2 KO mice, 82 ± 14% of control; n = 6). To verify whether recovery of renal Hnf1α would restore Sglt2 and whether re-expression of renal Glut2 would normalise renal Hnf1α as well as Sglt2, we transfected primary renal proximal tubular epithelial cells isolated from Ks-Glut2 KO mice (21 days after inducing renal Glut2 deficiency) with Hnf1α- or Glut2-expressing plasmids. By the third day of the transfections, Sglt2 was restored in Hnf1α-expressing Glut2-deficient cells, and both Sglt2 and Hnf1α were recovered in the cells transfected with Glut2 (Fig. 6j). These results demonstrate the contribution of renal Hnf1α to mediating the crosstalk between renal GLUT2 and SGLT2.
Discussion
Here, we have established the physiological contribution of renal GLUT2 to systemic glucose homeostasis using inducible Ks-Glut2 KO mice. The KO mice exhibited improved glucose tolerance independently of changes in insulin secretion or sensitivity. Moreover, the KO mice recovered from hyperglycaemia in a model of insulin-dependent diabetes within a week after inducing renal Glut2 deficiency. In a model of diet-induced insulin resistance, renal Glut2 deficiency normalised glucose tolerance and reversed obesity. We have also identified a novel crosstalk between renal GLUT2 and SGLT2 via the transcription factor HNF1α.
Although GLUT2 is a major glucose transporter in the kidneys, its contribution to systemic glucose homeostasis is not well defined. Previous reports [24, 26], including some from our laboratory [27, 28, 32], have indicated the therapeutic potential of blocking renal GLUT2 in improving blood glucose control through elevated glucosuria. We recently demonstrated that, despite obesity and insulin resistance, hypothalamus-specific Pomc and Mc4r KO mice exhibit improved glucose tolerance probably because of reduced renal GLUT2 and consequent elevated glucosuria [27, 28, 32]. Based on these findings, we produced Ks-Glut2 KO mice and assessed their glucose homeostasis in this current study. The KO mice exhibited massive glucosuria and improved glucose tolerance probably because of reduced glucose reabsorption owing to the lack of renal Glut2. Moreover, these mice exhibited normal circulating glucagon, NEFA and β-hydroxybutyrate. This phenotype strongly contrasts with that of global Glut2 KO mice, which show impaired insulin secretion and have elevated plasma levels of glucagon, NEFA and β-hydroxybutyrate [3]. These differences demonstrate the precise contribution of renal GLUT2 to systemic glucose homeostasis, and the lack of effect on other physiological variables that would otherwise have been changed due to global GLUT2 deficiency. Remarkably, the beneficial effects of renal Glut2 deficiency were abolished when we knocked out Glut2 in the liver in addition to the kidneys. These results support previous findings that hepatic Glut2 deficiency impairs glucose homeostasis [39]. Overall, our present study highlights the differential tissue-specific contribution of GLUT2 to systemic glucose homeostasis.
Diabetes increases renal GLUT2 in humans and rodents [19,20,21,22,23,24,25], an effect that we have also validated in this present study, thereby enhancing glucose reabsorption and worsening hyperglycaemia. Hence, inhibiting GLUT2 specifically in the kidneys would likely break this vicious cycle and could reverse hyperglycaemia by elevating glucosuria. Indeed, in the present study we observed that inducing Glut2 deficiency in the kidneys reversed hyperglycaemia in a mouse model of type 1 diabetes and normalised glucose tolerance as well as body weight in a mouse model of diet-induced obesity. These findings provide preclinical evidence that inhibiting renal GLUT2 can reverse diabetes and obesity.
SGLT2 inhibition is an established therapy for treating diabetes [13,14,15,16,17,18]. However, SGLT2 inhibitors reduce glucose reabsorption by only 30–50%, probably because of the compensation of SGLT1 in the S3 segment, accomplished by GLUT2 or GLUT1 in the basolateral membrane [11, 40,41,42]. Hence, inhibiting renal GLUT2 may overcome this limitation of SGLT2 inhibitors. Sglt2 KO mice exhibit massive glucosuria and improved glucose tolerance [12, 18]. In the present study, we show that Ks-Glut2 KO mice also display a similar degree of glucosuria and improved glucose tolerance, illustrating that renal GLUT2 is as significant as SGLT2 in influencing systemic glucose homeostasis by regulating glucosuria. SGLT2 inhibition increases circulating glucagon, β-hydroxybutyrate and NEFA, causing serious ketoacidosis [43,44,45]. However, we did not observe these changes or ketoacidosis in Ks-Glut2 KO mice. These contrasting results indicate that distinct neurohumoral pathways may be involved in either increasing glucose production or utilisation of alternative sources of energy in response to the loss of glucose in urine mediated by blocking the two different glucose transporters. Dapagliflozin further improved glucose tolerance and elevated glucosuria in Ks-Glut2 KO mice in the presence and absence of diabetes. These findings suggest that combined inhibition of renal GLUT2 and SGLT2 may yield optimal glucose control in diabetes.
Here, we have also identified a previously unknown crosstalk between renal GLUT2 and SGLT2. We have demonstrated that renal Glut2 deficiency in the long term almost abolished the expression of Sglt2 via downregulating the transcription factor HNF1α. This crosstalk may be clinically significant because HNF1α mutations in humans cause elevated glucosuria [36, 46] and monogenic diabetes [46, 47]. Previous studies have demonstrated that Hnf1α regulates Sglt2 expression [36,37,38]. Therefore, based on these reports and our findings from the current study, elevated glucosuria in individuals with HNF1α mutations could be attributed to HNF1α deficiency in the kidneys, and defective insulin secretion could be a result of the lack of HNF1α in beta cells [46]. The crosstalk between HNF1α and SGLT2 may also explain why SGLT2 inhibition produces a higher degree of glucosuria in individuals with HNF1α mutations than in those with type 2 diabetes [48]. Collectively, our present study indicates that genetic silencing of renal Glut2 would also almost eliminate the expression of Sglt2 by downregulating Hnf1α.
This study has some limitations. We did not comprehensively investigate why K+L Glut2 KO mice display impaired glucose tolerance despite elevated glucosuria. We did report that increased glucagon and defective GSIS in these mice could have contributed to their impaired glucose tolerance, although other developmental factors arising from hepatic Glut2 deficiency since birth may also have negatively affected glucose homeostasis in these mice. While we have demonstrated in the present study that loss of function of renal Glut2 in the long-term almost abolishes Sglt2 expression via downregulation of renal Hnf1α, it remains unclear whether the reverse is true (i.e. whether genetic Sglt2 deficiency would eliminate the expression of Glut2). Sglt2 null mice have normal renal Glut2 expression at a specific age [12] but this outcome may change over time based on our observation in the present study that renal Glut2 deficiency causes a progressive decrease in renal Sglt2 expression. Although we had observed that renal Glut2 deficiency reversed diet-induced obesity, precise mechanisms underlying this phenotype remain unclear. The loss of energy through elevated glucosuria is one contributing factor to reversing the obesity but other mechanisms [49,50,51] such as enhanced lipolysis, increased energy expenditure and/or reduced inflammation in normalising body weight in Ks-Glut2 KO mice need further investigation.
Altogether, findings from this study demonstrate that inhibiting renal GLUT2 can reverse hyperglycaemia and obesity in mice. Moreover, combined inhibition of renal GLUT2 and SGLT2 may achieve optimal blood glucose control in diabetes. We have also uncovered a novel crosstalk between renal GLUT2 and SGLT2 via the transcription factor HNF1α, which raises the possibility that some of the clinical features of Fanconi–Bickel syndrome may be secondary to the downregulation of HNF1α and SGLT2.
Data availability
All data are available in the main text or the supplementary materials. Additional raw data are available upon request to the corresponding author. The mouse models used in this study are available via material transfer agreement addressed to the corresponding author.
Abbreviations
- GSIS:
-
Glucose-stimulated insulin secretion
- HFSD:
-
High-fat + sucrose diet
- HNF1α:
-
Hepatocyte nuclear factor-1α
- KO:
-
Knockout
- Ks-Glut2 KO:
-
Glut2 knocked out specifically in the kidneys
- SGLT1:
-
Sodium–glucose cotransporter 1
- SGLT2:
-
Sodium–glucose cotransporter 2
- STZ:
-
Streptozotocin
- TBST:
-
Tris-buffered saline with 0.1% Tween 20 detergent
References
Thorens B, Cheng ZQ, Brown D, Lodish HF (1990) Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Phys 259(6 Pt 1):C279–C285. https://doi.org/10.1152/ajpcell.1990.259.2.C279
Thorens B, Sarkar HK, Kaback HR, Lodish HF (1988) Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and β-pancreatic islet cells. Cell 55(2):281–290. https://doi.org/10.1016/0092-8674(88)90051-7
Guillam MT, Hümmler E, Schaerer E et al (1997) Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet 17(3):327–330. https://doi.org/10.1038/ng1197-327
De Vos A, Heimberg H, Quartier E et al (1995) Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 96(5):2489–2495. https://doi.org/10.1172/jci118308
McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL (2011) GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol Genet Metab 104(4):648–653. https://doi.org/10.1016/j.ymgme.2011.08.026
Santer R, Schneppenheim R, Suter D, Schaub J, Steinmann B (1998) Fanconi-Bickel syndrome--the original patient and his natural history, historical steps leading to the primary defect, and a review of the literature. Eur J Pediatr 157(10):783–797. https://doi.org/10.1007/s004310050937
Santer R, Schneppenheim R, Dombrowski A, Götze H, Steinmann B, Schaub J (1997) Mutations in GLUT2, the gene for the liver-type glucose transporter, in patients with Fanconi-Bickel syndrome. Nat Genet 17(3):324–326. https://doi.org/10.1038/ng1197-324
Sansbury FH, Flanagan SE, Houghton JA et al (2012) SLC2A2 mutations can cause neonatal diabetes, suggesting GLUT2 may have a role in human insulin secretion. Diabetologia 55(9):2381–2385. https://doi.org/10.1007/s00125-012-2595-0
Khandelwal P, Sinha A, Jain V, Houghton J, Hari P, Bagga A (2018) Fanconi syndrome and neonatal diabetes: phenotypic heterogeneity in patients with GLUT2 defects. CEN Case Rep 7(1):1–4. https://doi.org/10.1007/s13730-017-0278-x
Beermann F, Thorens B, Guillam M-T, Burcelin R, Jaquet M (2000) Transgenic Reexpression of GLUT1 or GLUT2 in pancreatic β cells rescues GLUT2-null mice from early death and restores normal glucose-stimulated insulin secretion. J Biol Chem 275(31):23751–23758. https://doi.org/10.1074/jbc.M002908200
Ghezzi C, Loo DDF, Wright EM (2018) Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 61(10):2087–2097. https://doi.org/10.1007/s00125-018-4656-5
Vallon V, Platt KA, Cunard R et al (2011) SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22(1):104–112. https://doi.org/10.1681/asn.2010030246
Abdul-Ghani MA, DeFronzo RA (2008) Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 14(6):782–790. https://doi.org/10.4158/ep.14.6.782
DeFronzo RA, Hompesch M, Kasichayanula S et al (2013) Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36(10):3169–3176. https://doi.org/10.2337/dc13-0387
Santer R, Kinner M, Lassen CL et al (2003) Molecular analysis of the <em>SGLT2</em> gene in patients with renal Glucosuria. J Am Soc Nephrol 14(11):2873–2882. https://doi.org/10.1097/01.Asn.0000092790.89332.D2
Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA (1987) Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79(5):1510–1515. https://doi.org/10.1172/JCI112981
Oku A, Ueta K, Arakawa K et al (1999) T-1095, an inhibitor of renal Na+−glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 48(9):1794–1800. https://doi.org/10.2337/diabetes.48.9.1794
Vallon V, Rose M, Gerasimova M et al (2013) Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304(2):F156–F167. https://doi.org/10.1152/ajprenal.00409.2012
Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J (2005) Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non–insulin-dependent diabetes. Diabetes 54(12):3427–3434. https://doi.org/10.2337/diabetes.54.12.3427
Dominguez JH, Camp K, Maianu L, Feister H, Garvey WT (1994) Molecular adaptations of GLUT1 and GLUT2 in renal proximal tubules of diabetic rats. Am J Phys 266(2 Pt 2):F283–F290. https://doi.org/10.1152/ajprenal.1994.266.2.F283
Marks J, Carvou NJ, Debnam ES, Srai SK, Unwin RJ (2003) Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol 553(Pt 1):137–145. https://doi.org/10.1113/jphysiol.2003.046268
Kamran M, Peterson RG, Dominguez JH (1997) Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephrol 8(6):943–948. https://doi.org/10.1681/ASN.V86943
Chin E, Zamah AM, Landau D et al (1997) Changes in facilitative glucose transporter messenger ribonucleic acid levels in the diabetic rat kidney. Endocrinology 138(3):1267–1275. https://doi.org/10.1210/endo.138.3.5015
Hinden L, Udi S, Drori A et al (2018) Modulation of renal GLUT2 by the Cannabinoid-1 receptor: implications for the treatment of diabetic nephropathy. J Am Soc Nephrol 29(2):434–448. https://doi.org/10.1681/ASN.2017040371
Jiang Y-K, Xin K-Y, Ge H-W, Kong F-J, Zhao G (2019) Upregulation of renal GLUT2 and SGLT2 is involved in high-fat diet-induced gestational diabetes in mice. Diabetes Metab Syndr Obes 12:2095–2105. https://doi.org/10.2147/DMSO.S221396
Liu X-J, Wu X-Y, Wang H et al (2018) Renal injury in Seipin-deficient lipodystrophic mice and its reversal by adipose tissue transplantation or leptin administration alone: adipose tissue-kidney crosstalk. FASEB J 32(10):5550–5562. https://doi.org/10.1096/fj.201701427R
Chhabra KH, Adams JM, Fagel B et al (2016) Hypothalamic POMC deficiency improves glucose tolerance despite insulin resistance by increasing glycosuria. Diabetes 65(3):660–672. https://doi.org/10.2337/db15-0804
de Souza Cordeiro LM, Elsheikh A, Devisetty N et al (2021) Hypothalamic MC4R regulates glucose homeostasis through adrenaline-mediated control of glucose reabsorption via renal GLUT2 in mice. Diabetologia 64(1):181–194. https://doi.org/10.1007/s00125-020-05289-z
Lantinga-van Leeuwen IS, Leonhard WN, van de Wal A et al (2006) Transgenic mice expressing tamoxifen-inducible Cre for somatic gene modification in renal epithelial cells. Genesis 44(5):225–232. https://doi.org/10.1002/dvg.20207
Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ (2007) Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet 16(24):3188–3196. https://doi.org/10.1093/hmg/ddm299
Leonhard WN, Happe H, Peters DJM (2016) Variable cyst development in autosomal dominant polycystic kidney disease: the biologic context. J Am Soc Nephrol 27(12):3530–3538. https://doi.org/10.1681/asn.2016040425
Chhabra KH, Morgan DA, Tooke BP, Adams JM, Rahmouni K, Low MJ (2017) Reduced renal sympathetic nerve activity contributes to elevated glycosuria and improved glucose tolerance in hypothalamus-specific Pomc knockout mice. Mol Metab 6(10):1274–1285. https://doi.org/10.1016/j.molmet.2017.07.005
Ding W, Yousefi K, Shehadeh LA (2018) Isolation, characterization, and high throughput extracellular flux analysis of mouse primary renal tubular epithelial cells. J Vis Exp 136:57718. https://doi.org/10.3791/57718
Schreiber A, Shulhevich Y, Geraci S et al (2012) Transcutaneous measurement of renal function in conscious mice. Am J Physiol Renal Physiol 303(5):F783–F788. https://doi.org/10.1152/ajprenal.00279.2012
Albertoni Borghese MF, Majowicz MP, Ortiz MC, Passalacqua Mdel R, Sterin Speziale NB, Vidal NA (2009) Expression and activity of SGLT2 in diabetes induced by streptozotocin: relationship with the lipid environment. Nephron Physiol 112(3):p45–p52. https://doi.org/10.1159/000214214
Pontoglio M, Prié D, Cheret C et al (2000) HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep 1(4):359–365. https://doi.org/10.1093/embo-reports/kvd071
Freitas HS, Schaan BD, David-Silva A et al (2009) SLC2A2 gene expression in kidney of diabetic rats is regulated by HNF-1alpha and HNF-3beta. Mol Cell Endocrinol 305(1–2):63–70. https://doi.org/10.1016/j.mce.2009.02.014
Umino H, Hasegawa K, Minakuchi H et al (2018) High basolateral glucose increases sodium-glucose cotransporter 2 and reduces Sirtuin-1 in renal tubules through glucose Transporter-2 detection. Sci Rep 8(1):6791. https://doi.org/10.1038/s41598-018-25054-y
Seyer P, Vallois D, Poitry-Yamate C et al (2013) Hepatic glucose sensing is required to preserve β cell glucose competence. J Clin Invest 123(4):1662–1676. https://doi.org/10.1172/JCI65538
Powell DR, DaCosta CM, Gay J et al (2013) Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab 304(2):E117–E130. https://doi.org/10.1152/ajpendo.00439.2012
Chin E, Zhou J, Bondy C (1993) Anatomical and developmental patterns of facilitative glucose transporter gene expression in the rat kidney. J Clin Invest 91(4):1810–1815. https://doi.org/10.1172/jci116392
Liu JJ, Lee T, DeFronzo RA (2012) Why do SGLT2 inhibitors inhibit only 30-50% of renal glucose reabsorption in humans? Diabetes 61(9):2199–2204. https://doi.org/10.2337/db12-0052
Perry RJ, Rabin-Court A, Song JD et al (2019) Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor-treated rats. Nat Commun 10(1):548–548. https://doi.org/10.1038/s41467-019-08466-w
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB (2015) Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care 38(9):1687–1693. https://doi.org/10.2337/dc15-0843
Erondu N, Desai M, Ways K, Meininger G (2015) Diabetic ketoacidosis and related events in the Canagliflozin type 2 diabetes clinical program. Diabetes Care 38(9):1680–1686. https://doi.org/10.2337/dc15-1251
Pontoglio M, Sreenan S, Roe M et al (1998) Defective insulin secretion in hepatocyte nuclear factor 1alpha-deficient mice. J Clin Invest 101(10):2215–2222. https://doi.org/10.1172/jci2548
Yamagata K, Oda N, Kaisaki PJ et al (1996) Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature 384(6608):455–458. https://doi.org/10.1038/384455a0
Hohendorff J, Szopa M, Skupien J et al (2017) A single dose of dapagliflozin, an SGLT-2 inhibitor, induces higher glycosuria in GCK- and HNF1A-MODY than in type 2 diabetes mellitus. Endocrine 57(2):272–279. https://doi.org/10.1007/s12020-017-1341-2
Xu L, Nagata N, Nagashimada M et al (2017) SGLT2 inhibition by Empagliflozin promotes fat utilization and Browning and Attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine 20:137–149. https://doi.org/10.1016/j.ebiom.2017.05.028
Osataphan S, Macchi C, Singhal G et al (2019) SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 4(5). https://doi.org/10.1172/jci.insight.123130
Yokono M, Takasu T, Hayashizaki Y et al (2014) SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol 727:66–74. https://doi.org/10.1016/j.ejphar.2014.01.040
Acknowledgements
We thank the following people: J. LaFontaine, University of Alabama at Birmingham, for help with GFR measurements; G. Pryhuber, C. Poole and S. Mack, University of Rochester Medical Center Paediatric Histology Service (URMC-PHS), for help with histology; V. K. Thomas and J. Zhang, URMC Center for Advanced Light Microscopy and Nanoscopy, for help with microscopy; and K. Welle, University of Rochester Mass Spectrometry Resource Laboratory, for proteomics and [13C6]glucose assay.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
Funding sources are as follows: National Institutes of Health grant DK124619 (KHC); National Institutes of Health grant DK122190 (KHC); National Institutes of Health grant DK113115 (KHC); Startup funds and pilot research award, Department of Medicine, University of Rochester, NY (KHC); National Institutes of Health grant DK079337 (UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research); The Department of Paediatrics, University of Rochester, NY (URMC-PHS); National Institutes of Health grant HL148861(URMC-PHS); Pilot and feasibility award funded by the Pennington Biomedical NORC DK072476 (DHM). American Diabetes Association Junior Faculty Award 1-15-JF-37 (DHM); and National Institutes of Health instrument grant OD025242 to University of Rochester Mass Spectrometry Resource Laboratory.
Author information
Authors and Affiliations
Contributions
LMdSC and LB designed and performed experiments, analysed results, prepared graphs and figures, and edited the manuscript. ND genotyped the mice, performed experiments, and edited the manuscript. DHM provided Glut2loxP/loxP mice and edited the manuscript. DJMP provided KspCadCreERT2 mice and edited the manuscript. KHC conceived the study, designed and performed experiments, analysed results, prepared graphs and figures, and wrote and edited the manuscript. All the authors approved the final version of the manuscript. KHC is the guarantor of this work.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza Cordeiro, L.M., Bainbridge, L., Devisetty, N. et al. Loss of function of renal Glut2 reverses hyperglycaemia and normalises body weight in mouse models of diabetes and obesity. Diabetologia 65, 1032–1047 (2022). https://doi.org/10.1007/s00125-022-05676-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05676-8