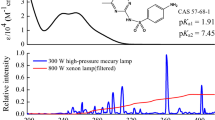
Abstract
The present study investigated the photolytic behavior and photodegradation products of mefenamic acid (MEF) under ultraviolet-C irradiation. The results demonstrated that the photodegradation of MEF followed pseudo-first-order kinetics and the direct photolysis quantum yield of mefenamic acid was observed to be 2.63 ± 0.28 × 10−3. Photodegradation of MEF included degradation by direct photolysis and by self-sensitization that the contribution rates of self-sensitized photodegradation were 5.70, 11.25 and 18.96 % for ·OH, 1O2 and \({\text{O}}_{2}^{ \cdot - }\), respectively. Primary transformation products of MEF were identified using ultra performance liquid chromatography and quadrupole time-of-flight mass spectrometer (UPLC-Q-TOF–MS). The identified transformation products suggested three possible pathways of MEF photodegradation: dehydrogenation, hydroxylation, and ketonized reactions. Toxicity of phototransformation products were evaluated using the Microtox test, which revealed that photodegradation likely provides a critical pathway for MEF toxicity reduction in drinking water and wastewater treatment facilities.







Similar content being viewed by others
References
Aranami K, Readman JW (2007) Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 66:1052–1056. doi:10.1016/j.chemosphere.2006.07.010
Boreen AL, Edhlund BL, Cotner JB, McNeill K (2008) Indirect photodegradation of dissolved free amino acids: the contribution of singlet oxygen and the differential reactivity of DOM from various sources. Environ Sci Technol 42(15):5492–5498. doi:10.1021/es800185d
Chen Y, Hu C, Qu J, Yang M (2008) Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J Photochem Photobiol A Chem 197:81–87. doi:10.1016/j.jphotochem.2007.12.007
Chen P, Lv WY, Chen ZM, Ma JS, Li RB, Yao K, Liu GG, Li FH (2015) Phototransformation of mefenamic acid induced by nitrite ions in water: mechanism, toxicity, and degradation pathways. Environ Sci Pollut Res. doi:10.1007/s11356-015-4537-0
Cogan S, Haas Y (2008) Self-sensitized photo-oxidation of para-indenylidene–dihydropyridine derivatives. J Photochem Photobiol A 193:25–32. doi:10.1016/j.jphotochem.2007.06.003
Fishbein JC, McClelland RA (1996) Halide ion trapping of nitrenium ions formed in the Bamberger rearrangement of N-arylhydroxylamines. Lifetime of the parent phenylnitrenium ion in water. Can J Chem 74:1321–1328. doi:10.1139/v01-178
Ge L, Chen J, Wei X, Zhang S, Qiao X, Cai X, Xie Q (2010) Aquatic photochemistry of fluoroquinolone antibiotics: kinetics, pathways, and multivariate effects of main water constituents. Environ Sci Technol 44(7):2400–2405. doi:10.1021/es902852v
Hilton MJ, Thomas KV (2003) Determination of selected human pharmaceutical compounds in effluent and surface water samples by high-performance liquid chromatography-electrospray tandem mass spectrometry. Chromatography 1015:129–141. doi:10.1016/S0021-9673(03)01213-5
Ji Y, Zeng C, Ferronato C, Chovelon JM, Yang X (2012) Nitrate-induced photodegradation of atenolol in aqueous solution: kinetics, toxicity and degradation pathways. Chemosphere 88:644–649. doi:10.1016/j.chemosphere.2012.03.050
Ji Y, Zhou L, Ferronato C, Yang X, Salvador A, Zeng C, Chovelon JM (2013) Photocatalytic degradation of atenolol in aqueous titanium dioxide suspensions: kinetics, intermediates and degradation pathways. J Photochem Photobiol A 254:35–44. doi:10.1016/j.jphotochem.2013.01.003
Jones OAH, Voulvoulis N, Lester JN (2002) Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res 36:5013–5022. doi:10.1016/S0043-1354(02)00227-0
Moll R, Derry S, Moore RA, McQuay HJ (2011) Single dose oral mefenamic acid for acute postoperative pain in adults. Cochrane Database Syst Rev. doi:10.1002/14651858
Sein MM, Zedda M, Tuerk J, Schmidt TC, Golloch A, Sonntag CV (2008) Oxidation of diclofenac with ozone in aqueous solution. Environ Sci Technol 42:6656–6662. doi:10.1021/es8008612
Soufan M, Deborde M, Legube B (2012) Aqueous chlorination of diclofenac: kinetic study and transformation products identification. Water Res 46:3377–3386. doi:10.1016/j.watres.2012.03.056
Suwalsky M, Manrique-Moreno M, Howe J, Brandenburg K, Villena F (2011) Molecular interactions of mefenamic acid with lipid bilayers and red blood cells. J Braz Chem Soc 22:2243–2249. doi:10.1590/S0103-50532011001200002
Tauxe-Wuersch A, De Alencastro LF, Grandjean D, Tarradellas J (2005) Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res 39:1761–1772. doi:10.1016/j.watres.2005.03.003
von Sonntag C (2006) Free-radical-induced DNA damage and its repair. Springer, Berlin
Werner JJ, McNeill K, Arnold WA (2005) Environmental photodegradation of mefenamic acid. Chemosphere 58:1339–1346. doi:10.1016/j.chemosphere.2004.10.004
Yamamoto H, Nakamura Y, Moriguchi S, Nakamura Y, Honda Y, Tamura I, Sekizawa J (2009) Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res 43:351–362. doi:10.1016/j.watres.2008.10.039
Yang X, Chen F, Meng F, Xie Y, Chen H, Young K, Fu W (2013) Occurrence and fate of PPCPs and correlations with water quality parameters in urban riverine waters of the Pearl River Delta, South China. Environ Sci Pollut Res 20(8):5864–5875. doi:10.1007/s11356-013-1641-x
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21377031), the Scientific and Technical Projects of Guangdong Province (No. 2013B020800009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, P., Wang, F.L., Yao, K. et al. Photodegradation of Mefenamic Acid in Aqueous Media: Kinetics, Toxicity and Photolysis Products. Bull Environ Contam Toxicol 96, 203–209 (2016). https://doi.org/10.1007/s00128-015-1680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1680-8