Abstract
Objective
Sedation by the enteral route is unusual in intensive medicine. We analysed the feasibility/efficacy of long-term enteral sedation in ventilated critically ill patients.
Design
Prospective interventional cohort study.
Setting
General ICU.
Patients and participants
Forty-two patients needing ventilation and sedation for at least 4 days.
Interventions
At admission, sedation was induced with propofol or midazolam. Enteral hydroxyzine (± enteral lorazepam) was added in all patients within the second day. Intravenous drugs were gradually withdrawn, trying to maintain only enteral sedation after the initial 48 h. Analgesia was provided with continuous IV fentanyl.
Measurements and results
Sedation level was assessed evaluating, on a daily basis, patients’ compliance to the invasive care and comparing observed vs planned Ramsay scores three times a day. Excluding the first 2 days of patient-stabilisation and fast titration of sedation level, 577 days with ventilatory support were analysed. In 460 days (79.7%) total enteral sedation was given. This percentage rose to 94.2% when the requested Ramsay was 2 (347 days). Daily sedation was judged as adequate in 82.8% of days of total enteral sedation. Thirty-one patients had total enteral as the exclusive route of sedation.
Conclusions
After 24–48 h, enteral sedation may replace, totally/in part, IV sedation in ventilated patients. Total enteral sedation easily fits the target when a Ramsay score 2 is planned. When a deeper sedation is needed, a mixed regimen is effective and lowers IV drug dosages. No side effects were reported.
Similar content being viewed by others
Introduction
Several guidelines [1, 2, 3] define the practice of sedation and analgesia in critically ill patients. Intravenous propofol, midazolam, and lorazepam are the drugs of choice in almost every situation.
Surprisingly, despite the feasibility of early gut feeding even in extreme conditions [4, 5], guidelines restrain the use of enteral sedatives to sleep induction in non-ventilated patients [3]. Nevertheless, after the first days of intensive care (ICU) stay, when most of the invasive procedures are performed and the requested level of sedation can abruptly/frequently change, the enteral route might maintain a stable level of sedation reducing the complexity of parenteral treatment and substantially abating costs.
We prospectively analysed the feasibility and efficacy of enteral sedation selecting very critically ill patients undergoing prolonged invasive assistance of vital functions. The results were previously reported in abstract form [6].
Methods
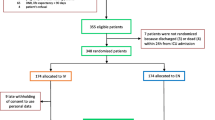
The study was conducted in a 6-bed general ICU from September 2000 to July 2001. The ethics committee approved the protocol and patients/next-of-kin gave informed consent. Consecutive patients were selected at admission on the basis of expected ventilatory assistance and sedation for at least 4 days. Exclusion criteria were age below 18 years, major gut resections, multiple fistulas and difficult evaluation of the sedation level (coma or curarisation). Demographic, clinical data, and severity score [7] were collected.
During the first 2 days of ICU stay, an infusion of propofol (0.5 mg/kg followed by 0.5–3 mg·kg·h) or midazolam (30–300 μg/kg followed by 30–200 μg·kg·h) was allowed. The choice was left to the physician in charge. Intravenous fentanyl at 1–2 μg·kg·h was given according to need. In this period enteral sedation was started as soon as possible with hydroxyzine. Since no indications exist on long-term enteral use in critically ill patients, our planned dosage (6–12 mg·kg·day in three doses) took into account the long half-life, and renal/organs function. Moreover, doses were titrated according to sedation end-points. Supplemental enteral lorazepam was allowed to optimise hydroxyzine sedation.
After the initial 48 h the target was to warrant the planned level of sedation, reducing IV while maintaining enteral treatment to realize a mixed or pure enteral sedation. Intravenous drugs not withdrawn within the following 2 days were replaced with IV lorazepam (10–30 μg·kg·h) as later supported by guidelines [3]. Enteral haloperidol was the drug of choice if delirium ensued. Boluses of sedatives, given when performing invasive procedures, were not taken into account in data analysis.
Each morning the physician and the nurse in charge planned the daily level of sedation (Ramsay scale [8]) on clinical grounds and patient characteristics. The preferred end-point was a tranquil, collaborative patient while intensively treated. Three times a day the sedation level was evaluated and, if necessary, titrated to patient’s need. Adequate sedation was defined as the achievement of the planned level (Ramsay score). Moreover, at the end of each day the nurse in charge subjectively defined the whole daily sedation as “adequate”, “insufficient” or “excessive” according the patients’ behaviour in the previous 24 h (signs of anxiety, agitation, pain, compliance with nursing, and patient/ventilator interaction). The efficacy of sedation on the days spent on ventilator (24 h), was evaluated from the third day of ICU stay (scheduled shift from IV to total enteral sedation). hydroxyzine side effects (constipation, diarrhoea, vomiting, blurred vision, urinary retention, renal, and hepatic damage) were monitored.
Data are reported as mean±standard deviation (SD), median, interquartile range (IQR). Pearson’s χ2 test and Student’s t-test were used for data comparisons.
Results
Two hundred and thirty-eight patients were consecutively admitted in 10 months. Among the 66 patients who received >4 days of ventilation and sedation, 24 were not enrolled: ten because the period of ventilation was underestimated at ICU admission, the remaining because of age (three), coma/curarisation (eight) or huge gut resection (three). Forty-two patients were enrolled, their demographic and clinical characteristics at admission are reported in Table 1.
Altogether, 24 patients (57.1%) had shock (septic in 17 patients). Seven (16.7%) underwent open abdominal treatment. The ICU length of stay was 22.1±23.6 days, median 13.5 (IQR 8–28). ICU and hospital mortality rates were 23.8% and 30.1%, respectively. All patients initially received ventilatory assistance through a naso-tracheal tube. Within a few days, a tracheostomy was performed in 23 of them. A naso-jejunal tube was positioned in 11 patients, a jejunostomy in two. The remaining patients had a naso-gastric tube.
Sedation was given in 701 days, 661 of them with ventilatory assistance: 15.7±15.6 days of ventilation per patient, median 10 (IQR 5–20). Enteral sedation was started during the admission day in 36 patients and in all within the second day. At that time, 37 patients were receiving enteral nutrition at a mean rate of 511±198 kcal/day.
Thirty-five patients were shifted to total enteral sedation within 72 h and further three within the fourth day. The remaining four patients, admitted for acute respiratory failure in pneumonia (two), hemorrhagic shock after major trauma and septic shock, continued a mixed sedation for 7–22 days. During ICU stay, seven patients resumed continuous IV sedation: all had septic shock and four open abdominal treatment. The 11 patients resuming (seven) or continuing (four) continuous IV sedation had a longer ventilation (30.8±21.3 vs 10.4±8.2 days; P=0.000) and a trend to higher ICU mortality rate (6/11 vs 4/31; P<0.094).
The sedative dosages in the whole 661 ventilated days are reported in Table 2. The 11 patients with prolonged IV/mixed sedation needed higher dosages of midazolam (76.3±76.0 vs 30.5±26.1 μg·kg·h, P=0.000), propofol (1.34±0.86 vs 0.92±0.64 mg·kg·h, P=0.049), hydroxyzine (10.1±3.7 vs 5.9±3.1 mg·kg·day, P=0.008) and enteral lorazepam (47.7±24.5 vs 27.4±18.0 μg·kg·day, P=0.000). No tachiphylaxis for hydroxyzine was observed.
Continuous IV fentanyl was given in 35 patients (since admission in 31 cases) for 6.2±8.0 days at the mean dose 1.00±1.18 μg·kg·h. Opiates were more used in surgical/trauma patients than in medical patients (141/349 days vs 52/228; P=0.002) and in patients who died than in ICU survivors (83/158 days, vs 110/419; P=0.000). Enteral haloperidol was given in two cases. Supplemental IV boluses of sedatives were administered 36 times during enteral sedation and 29 during IV/mixed sedation.
The requested Ramsay scores for the first 48 h (84 days) and for the following 577 days of ventilatory support are reported in Table 3. After the first two days, total enteral sedation was given in 460 (79.7%), mixed sedation in 101 (17.5%) and only IV drugs in 16 days (2.8%). Fentanyl was used in 118 out of the 460 days (25.7%) of pure enteral sedation, at a mean dose of 0.80±0.51 μg·kg·h, and in 79/117 days (67.5%) of IV/mixed sedation, at a mean dose of 1.11±0.60 μg·kg·h. Moreover, total enteral sedation was used in 94.2% of the 347 days in which a Ramsay score of 2 was requested and in 57.8% of the 230 days in which a deeper sedation was scheduled (P=0.000).
The overall daily adequacy of sedation and the three-times-a-day comparison between observed and planned Ramsay scores are reported in Table 4. Total enteral sedation was insufficient in 8.9% of the days with a planned Ramsay score of 2 at variance with 13.5% of the days in which a deeper sedation was planned. Patients that continued or resumed IV sedation needed a deeper sedation: a Ramsay score of 3 or higher was requested in 54.3% of their days compared to 23.5% in the remaining group, and IV/mixed sedation was used in 33.4% of their ventilated days compared to 4.2%.
Twenty-two patients out of 32 survivors (68.8%) were weaned in the ICU (six patients were transferred to other ICUs and four to step-down respiratory units while still ventilated). Fifteen (68.2%) of them resumed spontaneous breathing while receiving enteral sedation (eight hydroxyzine at 6.4±3.5 mg·kg·day and lorazepam at 24.7±19.5 μg·kg·day, five only hydroxyzine and two only lorazepam at lower dosages). These 22 patients were weaned in the ICU and their reintubation rate was null.
No major side effects, nor alterations in hepatic and renal functions specifically related to hydroxyzine were reported. Doses never had to be modified because of hemodynamic impairment.
Discussion
Sedation and analgesia are mandatory in reducing the stress response, in facilitating ventilation, in increasing tolerance to ICU procedures, and in preventing self-removal of invasive devices [9, 10, 11]. Sedative drugs are usually given by vein for a fast titration of the sedation level. However, after the first 24–48 h of patient’s initial stabilization, few authors used enteral sedation [12, 13, 14, 15] despite the evidence that the gut can be used for early nutrition [4, 5].
By measuring its efficacy, we tested the feasibility of the enteral route, alone or in combination with IV drugs in the sedation of the most intensively treated patients (at least 4 days of ventilatory assistance preconized at ICU admission), in their most critical phase of care by selecting only the ventilation period (Table 1). Due to our selection criteria, we excluded the less complex ICU population, i.e., the majority (78%) of the observed case-mix.
Even if different enteral sedative drugs might have been chosen, we used hydroxyzine because of potent anxiolytic with minor cardio-respiratory effects [16] adding lorazepam to optimise and tuning the sedation level. Moreover, analgesia, when needed, was provided with fentanyl. Enteral sedation was started in all patients within the first 48 h, even in the five cases in which enteral nutrition had to be delayed, and mainly through the gastric route. The shift to pure enteral sedation was possible in the great majority of patients within the fourth day, and was definitive in 31 of them. Altogether, pure enteral sedation covered 80% of the ventilated days after the first two and, was judged as sufficient or even excessive, in about 90% of the assessments (Table 4).
A small subset of patients, probably the most complicated (most of them had shock and sepsis and showed longer duration of ventilation and ICU stay and higher mortality rate), had to continue or resume IV sedation. They needed a deeper sedation which was obtained with a mixed sedative regimen. An impaired gut perfusion affecting drug absorption might also have been present.
This study has some limits: a) patient selection criteria were quite restrictive, unusual, and based on clinical prediction. Nevertheless, we enrolled 81% of the our eligible patients (after applying exclusion criteria); b) lack of a control group hinders us from knowing if pure enteral or mixed sedation has any advantage on pure IV sedation regarding side effects, length of ICU stay, days of ventilation, cardiovascular stability, patient’s recall, and global costs; c) the long half-life of hydroxyzine coupled with long-term usage and the presence of variable organ failure may result in some degree of accumulation. This problem was managed titrating sedation to the minimum level able to obtain the planned sedation level.
A few final considerations can be drawn. Our study shows that pure enteral sedation is feasible and effective when the team accepts as a target of sedation a tranquil, cooperative, pain-free patient (Ramsay score of 2).The majority of our invasively treated patients, though selected from among the most complicated at ICU admission, were successfully maintained at this level of sedation during the whole ventilation period (Table 3, Table 4). Analgesic drugs did not have a role in determining the use of pure enteral sedation. In fact, fentanyl was used more frequently and at similar doses in IV/mixed than in pure enteral sedation days. All the more reason that we assume that enteral sedation can similarly work in the less complex patients who represent the majority of the ICU population. Nonetheless, pure enteral sedation may be ineffective in case of impaired gut perfusion or when a deeper sedation is deemed advisable by the ICU team. However, in the latter scenario, enteral sedation is still effective in more than half of the cases and allows the use of reduced IV drugs dosages in mixed sedation days.
Regarding the effect on the duration of ventilation [17, 18, 19], an adverse impact of enteral sedation may be lessened, the majority of our weaned patients were still receiving enteral sedation at suspension of ventilatory support.
Finally, IV sedation is quite expensive. The drug cost of a full dose of pure enteral sedation is significantly lower (10–15 times) than the cost of a full dose of IV sedation. Mixed sedation may also have a substantial cost saving effect, due to reduced doses of IV drugs.
References
Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ (2000) Sedation in the Intensive Care unit: a systematic review. JAMA 283:1451–1459
Izurieta R, Rabatin JT (2002) Sedation during mechanical ventilation: a systematic review. Crit Care Med 30:2644–2648
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, Crippen DW, Fuchs BD, Kelleher RM, Marik PE, Nasraway SA Jr, Murray MJ, Peruzzi WT, Lumb PD (2002) Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 30:119–141
Iapichino G, Pesenti A, Radrizzani D, Solca M, Pelizzola A, Gattinoni L (1983) Nutritional support to long term anaesthetized and curarized patients under extracorporeal respiratory assist for terminal pulmonary failure. JPEN 7:50–54
Revelly JP, Tappy L, Berger MM, Gersbach P, Cayeux C, Chiolero R (2001) Early metabolic and splanchnic responses to enteral nutrition in postoperative cardiac surgery patients with circulatory compromise. Intensive Care Med 27:540–547
Cigada M, Pezzi A, Assi E, Borotto E, Giacomini M, Marzorati S, Iapichino G (2003) Enteral sedation in the critical patient. Intensive Care Med 29 [Suppl 1]:S24
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPSII) based on a European-North American multicenter study. JAMA 270:2957–2963
Ramsay MA, Savege TM, Simpsom BR, Goodwin R (1974) Controlled sedation with alphaxalone-alphadolone. Br Med J 2:656–659
Mazzeo AJ (1995) Sedation for the mechanically ventilated patient. Crit Care Clin 11:937–955
Durbin CG Jr (1996) Sedation in the critically ill patient. New Horizons 2:64–74
Joint Commission on Accreditation of Healthcare Organizations (JCAHO) (2001) Comprehensive accreditation manual for long term care. Standards and intentions for sedation and anaesthesia care. Revisions to anaesthesia care standards, effective January 1, 2001
Iapichino G, Borelli M, Breda G, Ciceri R, Ferrarsi S, Passoni M (1989) Enteral polypharmacological sedation management in critical patients. Anestesia e Rianimazione 30:75–78
Lugo RA, Chester EA, Cash J, Grant MJC, Vernon DD (1999) A cost analysis of enterally administered lorazepam in the paediatric intensive care unit. Crit Care Med 27:417–421
Perez JE, Nash JE (1999) Continuous enteral lorazepam in the trauma patient. Chest 116 [Suppl 2]:278S
Arenas-Lopez S, Riphagen S, Tibby SM, Durward A, Tomlin S, Davies G, Murdoch IA (2004) Use of oral Clonidine for sedation in ventilated paediatric intensive care patients. Intensive Care Med 30:1625–1629
Lauria JI, Markello R, King BD (1968) Circulatory and respiratory effects of hydroxyzine in volunteers and geriatric patients. Anesth Analg Curr Res 47:378–382
Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G (1998) The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest 114:541–548
Brooks AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH (1999) Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 27:2609–2615
Kress JP, Pohlman AS, O’Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342:1471–1477
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cigada, M., Pezzi, A., Mauro, P.D. et al. Sedation in the critically ill ventilated patient: possible role of enteral drugs. Intensive Care Med 31, 482–486 (2005). https://doi.org/10.1007/s00134-005-2559-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2559-7