Abstract
Purpose
To examine the impact of different endotracheal tube (ETT) suction techniques on regional end-expiratory lung volume (EELV) and tidal volume (V T) in an animal model of surfactant-deficient lung injury.
Methods
Six 2-week old piglets were intubated (4.0 mm ETT), muscle-relaxed and ventilated, and lung injury was induced with repeated saline lavage. In each animal, open suction (OS) and two methods of closed suction (CS) were performed in random order using both 5 and 8 French gauge (FG) catheters. The pre-suction volume state of the lung was standardised on the inflation limb of the pressure-volume relationship. Regional EELV and V T expressed as a proportion of the impedance change at vital capacity (%Z VCroi) within the anterior and posterior halves of the chest were measured during and for 60 s after suction using electrical impedance tomography.
Results
During suction, 5 FG CS resulted in preservation of EELV in the anterior (non-dependent) and posterior (dependent) lung compared to the other permutations, but these only reached significance in the anterior regions (p < 0.001 repeated-measures ANOVA). V T within the anterior, but not posterior lung was significantly greater during 5FG CS compared to 8 FG CS; the mean difference was 15.1 [95% CI 5.1, 25.1]%Z VCroi. Neither catheter size nor suction technique influenced post-suction regional EELV or V T compared to pre-suction values (repeated-measures ANOVA).
Conclusions
ETT suction causes transient loss of EELV and V T throughout the lung. Catheter size exerts a greater influence than suction method, with CS only protecting against derecruitment when a small catheter is used, especially in the non-dependent lung.
Similar content being viewed by others

Introduction
Maintaining adequate lung volume is essential to optimising gas exchange [1–4] and minimising ventilator-induced lung injury [5–9]. However, infants receiving mechanical ventilation are frequently exposed to lung derecruitment during suction of the endotracheal tube (ETT) [10, 11]. Closed suction (CS), which, in principle, allows tidal ventilation to continue, has been recommended as a method of minimising derecruitment and deoxygenation [11, 12]. Nevertheless, it is clear that loss of end-expiratory lung volume can occur during CS in mechanically ventilated infants and children [10, 11, 13, 14].
Quantifying the impact of ETT suction on the lung has been hampered by a lack of reliable bedside tools to measure changes in lung volume and mechanics. Most studies of lung derecruitment following ETT suction have been limited to global measures of end-expiratory lung volume (EELV) [10, 11, 13, 15]. Such measurements cannot discern regional differences in lung behaviour, at the very least related to greater gravity-related superimposed pressure applied to the dependent regions of the lung [16]. In adult animal studies of ETT suction, these regions are prone to greater derecruitment than the non-dependent regions, with resultant post-suction ventilation inhomogeneity [17]. This investigation highlighted not only the regional changes in volumetric behaviour within the lung during suction, but also exemplifies the capacity of electrical impedance tomography (EIT) to measure such changes. EIT has been used to measure regional changes in EELV and tidal ventilation in paediatric and adult lung disease and neonatal animal studies [17–23], including studies of ETT suction [14, 23].
The aim of this study was to examine the impact of ETT suction on regional lung mechanics and volume in an animal model of surfactant-deficient lung injury. We investigated whether suction catheter size or method of suction (closed versus open) impacts upon the degree of regional lung volume loss and ventilation inhomogeneity.
Method
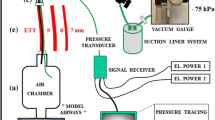
The study was approved by our institutions Animal Ethics Committee. Two-week-old male piglets (n = 6) were anaesthetised, intubated with a 4.0 cuffed ETT, muscle-relaxed and ventilated in supine position with time-cycled pressure limited ventilation (Bear Cub®, Viasys Healthcare, Yorba Linda, CA) using a protocol we have described previously [15]. Lung injury was induced using repeated saline lavage until the alveolar-arterial oxygen difference (AaDO2) was >400 mmHg. Ventilation thereafter continued with an inspiratory time of 0.5 s, positive end-expiratory pressure (PEEP) 5 cmH2O and inspired oxygen fraction 1.0. Peak inspiratory pressure (PIP) was adjusted to maintain tidal volume (V T) of 6–10 ml/kg and achieve an arterial partial pressure of CO2 35–45 mmHg at a rate of 30 breaths per minute.
Airway opening pressure was measured with the Florian respiratory monitor (Acutronic Medical Systems, Zug, Switzerland). Changes in lung impedance were measured using EIT (GeoMF II system, Cardinal Health, Hoechberg, Germany) [20]. A DC-coupled respiratory inductive plethysmograph (RIP; Respitrace 200, Non-invasive Monitoring Systems Inc., North Bay Village, FL) was used to measure global lung volume for the purpose of targeting a pre-determined point on the pressure–volume (PV) relationship before each suction episode [2, 15].
Mapping of the pressure-volume relationship
Prior to commencement of the experimental series, the PV relationship of the lung was mapped as previously described [15], using PIP and PEEP increments and decrements of 5 cmH2O, preceded by transient disconnection to ambient pressure to register residual volume. Plethysmographic (RIP) lung volume data were recorded and displayed continuously throughout the manoeuvre, using a custom-built data acquisition program designed in Labview 6.0™ (National Instruments, Austin, TX). Total lung capacity (TLC) was identified as the point beyond which no further increase in EELV occurred; vital capacity (VC) was defined as the difference between TLC and residual volume. EIT recordings were made concurrently, allowing the regional impedance change for VC (relative ΔZ VC in dimensionless units) to be determined in the same manner. The global PV curve was displayed during the experimental series as a reference for lung volume standardisation prior to each suction episode (see below).
ETT suction protocol
Suction episodes using all permutations of three suction methods (open, closed inline and closed using a side-port adapter) and two catheter sizes (5 and 8 FG; external diameter 1.65 and 2.64 mm) were performed in random order. CS was performed both with the Ballard Trachcare™ inline system (Kimberly-Clark, Roswell, GA) and the Neo-LINK™ universal side-port adaptor (Viasys MedSystems, Wheeling, IL), which contains a self-sealing valve that permits catheter entry while preventing circuit leak. Disposable catheters, pre-measured to the ETT tip, with an end hole and two side holes (Mallinkrodt, Rowville, Victoria, Australia) were used for open and side-port adaptor suction. In all cases a suction pressure of −140 mmHg was applied for 6 s while withdrawing the catheter, the typical practice in our institutions. No recruitment manoeuvres were performed after suction. The times at which the catheter was advanced into the circuit, negative pressure applied and the catheter completely withdrawn from the circuit were manually recorded. Prior to each suction episode, pre-suction EELV was standardised to a lung volume half-way between residual volume and TLC on the inflation limb by incremental manipulations of PEEP after transient disconnection to atmosphere, and tidal ventilation thereafter continued at this volume state for 10 min prior to each suction episode. Stability of EELV before suction was confirmed using the RIP volume trace.
Data collection and analysis
Changes in lung impedance were recorded before, during and for 1-min after suction using EIT, sampling at 13 Hz. For data acquisition and reconstruction of functional EIT data, the software provided with the equipment was used (SCIEIT V8, University of Göttingen, Göttingen, Germany) [24, 25]. EIT data were then analysed offline using purpose written software (MATLAB® V7.1, MathsWorks, Natick, MA). For each recording a frequency distribution of the EIT signal was constructed using fast Fourier transformation and a band-pass filter applied between 0 and 100 cycles per minute to isolate the ΔZ within the respiratory domain.
Changes in regional EELV and V T
EIT images were divided into two regions of interest: the dependent (posterior) and the non-dependent (anterior) lung. For each region the time course impedance signal was obtained for the entire suction procedure. During each tidal inflation, EELV was defined as the trough impedance value and V T as the tidal amplitude. From each recording, key phases were identified using the pressure signal and time data (Fig. 1): pre-suction, passing of suction catheter through the ETT, point of maximum EELV loss during the application of negative pressure and at 10 s intervals for 60 s post-suction. Regional EELV and V T impedance (Z EELV and Z VT) were determined at each time point, and expressed as a proportion of the Z VC within that region (%Z VCroi). To allow for comparison ΔZ EELV and ΔZ VT during and after suction were referenced to the respective pre-suction values. For instance, a ΔEELV of 20% Z VCroi means an increase in EELV by an amount equal to 20% of the VC for that region. As OS involved disconnection from mechanical ventilation, no V T data were obtained during suction by this method.
Illustrative EIT recording of change in global impedance (ΔZ) during a representative episode of ETT suction (closed inline suction with an 8 FG catheter) to demonstrate the key time points at which regional ventilation characteristics were calculated: a pre-suction; b passage of suction catheter during ventilation (CS) or after disconnection (OS); c minimum EELV during application of negative pressure; post-suction recovery after reinstitution of uninterrupted tidal ventilation with parameters determined over three consecutive inflations at 10-s intervals (dashed lines). Resultant functional impedance tomogram at each time point is also illustrated (see Electronic Supplementary Material)
Regional relative ΔEELV and ΔV T for each suction episode were compared using repeated measures ANOVA with Bonferroni post-test analysis, or paired t tests as applicable. Statistical analysis was performed using GraphPad Prism V4.02 (Graphpad, San Diego, CA), and p < 0.05 was considered significant.
Results
The six animals had a mean (SD) weight of 5.1 (0.4) kg and AaDO2 of 572 (78) mmHg after lavage. The median (inter-quartile range; IQR) PIP and PEEP were 28 (25.5, 28.5) and 7.0 (5.5, 8.5) cm H2O respectively, after obtaining VC at a PEEP of 32.5 (20.0, 40.0) cm H2O. The same PIP and PEEP were used for each suction episode for each animal. At commencement of the suction permutations, the median (IQR) peripheral oxygen saturation, mean arterial blood pressure and V T were 94 (87.5, 96.5)%, 83.5 (71, 98.5) cm H2O and 11.2 (10.3, 12.1) ml/kg, respectively. All animals completed the study protocol. For all parameters analysed there was no difference in the two closed suction methods. Thus, to simplify subsequent results, the CS data have been combined.
Effect of suction on regional EELV
Figure 2 shows the regional ΔEELV by suction method and catheter size. A similar pattern of EELV loss occurred during suction using all but CS with the 5 FG catheter (Table 1). Within the anterior region, both insertion of the suction catheter and subsequent application of negative pressure (maximum EELV loss) resulted in a significantly lower EELV using 5 FG OS and both OS and CS 8 FG compared to pre-suction and all post-suction time points (all p < 0.001 repeated measures ANOVA post-test analysis). Whilst a similar loss of EELV was also observed within the posterior region during CS 8 FG and OS with both catheter sizes, these differences were not significant. ΔEELV did not change significantly in either region during CS with a 5 FG catheter (see Electronic Supplementary Material). Within both the anterior and posterior chest there was no difference in the ΔZ EELV during insertion and at the point of minimum EELV using CS 8 FG and both OS (repeated measures ANOVA). All resulted in significantly lower ΔZ EELV within each region compared to CS 5 FG (p < 0.0001; repeated measures ANOVA with post-test analysis).
Change in relative end-expiratory lung volume impedance (ΔZ EELV), expressed as a ratio of the vital capacity ΔZ (%Z VCroi) and referenced to pre-suction Z EELV, in the posterior (closed circles and dashed lines) and anterior (open diamonds and solid lines) halves of the chest during open (a 5 FG catheter; b 8 FG catheter) and closed suction (c 5 FG; d 8 FG). Within the anterior lung regions the ΔZ EELV during insertion of the suction catheter (†) and at the point of minimum EELV (‡) was significantly less during 5 FG closed suction compared to 8 FG closed suction and open suction with both catheter sizes (p < 0.0001; repeated measures ANOVA with Bonferroni post-test analysis). Minimum EELV was significantly lower than pre-suction and 60 s post-suction values using open (5 FG and 8 FG) and closed 8 FG suction (all p < 0.001 repeated measures ANOVA post-test analysis). All data mean ± SD
For each suction permutation, ΔEELV at each time point was similar within both regions (repeated measures ANOVA post-test analysis). After suction regional EELV rapidly recovered in all cases, with no significant differences between permutations at any time point during the 60 s. In all cases ΔZ EELV 60 s post-suction did not differ from pre-suction values (ANOVA post-test analysis).
Effect of suction on regional VT
Figure 3 shows the regional behaviour of V T during OS and CS. By definition no V T occurred during OS. CS with an 8 FG catheter caused a significantly greater loss of V T within the anterior region during the application of suction compared to the 5 FG catheter; ΔZ VT mean [95% CI] difference 15.1 [5.1, 25.1] %Z VCroi, paired t test. A similar reduction in V T occurred in the posterior chest but the ΔZ VT mean [95% CI] difference of 9.7 [−0.3, 19.6] %Z VCroi did not reach significance. After suction, V T recovered quickly within all lung regions, irrespective of suction method or catheter size. For all permutations ΔZ VT 10 s after suction did not differ from the pre-suction value (repeated measure ANOVA). There was no difference in the regional recovery of V T for each permutation.
Change in relative tidal volume (ΔZ VT), expressed as a ratio of the vital capacity ΔZ (%Z VCroi) and referenced to pre-suction Z VT, in the posterior (closed circles and dashed lines) and anterior (open diamonds and solid lines) halves of the chest during open (a 5 FG catheter; b 8 FG catheter) and closed suction (c 5 FG; d 8 FG). ΔZ VT was greater in the anterior half of the chest during CS with an 8 FG compared to the 5 FG catheter (†p = 0.0016; paired t test). ΔZ VT was also greater in the posterior chest with the 8FG catheter, but did not reach significance (p = 0.076). ΔZ VT had recovered to pre-suction values within both lung regions by 10 s of completing suction in all four permutations of suction method and catheter size. All data mean ± SD
Discussion
Whilst many studies have investigated optimisation of lung volume using recruitment manoeuvres, there are few reports of the impact of suction on lung volume. This is surprising as ETT suction is one of the most common interventions performed on ventilated patients [26]. In this study, we demonstrated that regional changes in EELV and ventilation were influenced by suction technique, albeit transiently, in an animal model of lung injury. Suction catheter size exerted a greater influence on regional changes in ventilation than method of suction. The study is also the first to our knowledge to systematically describe regional tidal ventilation during and after ETT suction.
Gravity-related regional differences in lung volume and ventilation are known to exist in the diseased lung [16, 27]. These are especially prominent when ventilation is applied along the inflation limb of the PV relationship [27]. In our study ventilation was applied on the inflation limb, and the regional changes in EELV and V T observed during suction were relatively uniform for each suction permutation. In the case of OS and CS with the 8 FG catheter, these losses were between 33 and 50% of vital capacity within that lung region. In contrast, in an adult pig study, loss of EELV was greater in the dependent lung during suction [17], whilst Wolf and co-workers observed greater EELV loss within the non-dependant lung regions during CS in paediatric patients [14]. Our study differed from these in a number of important aspects that may explain these inconsistencies. Firstly, in the study of Lindgren and co-workers [17], regional changes in EELV were expressed relative to the global ΔZ. This assumes ΔZ measurement to be comparable between lung regions; this assumption has recently been questioned [28, 29]. By contrast, in our study regional ΔEELV and ΔV T were expressed in proportion to the vital capacity for that lung region, recognising that VC, and therefore the potential for impedance change, may differ between regions. Secondly, the pre-suction volume state of the lung was standardised in our study, allowing comparative assessments of relative regional changes in EELV and V T. We argue that, where feasible, standardising the volume state should be a prerequisite in future studies of regional lung derecruitment.
The loss of EELV during ETT suction has been attributed to the loss of PEEP and interruption to tidal ventilation, and CS has been advocated as a method of preserving both [11, 30, 31]. We have shown previously that CS only preserves global EELV when using a small suction catheter relative to the internal diameter of the ETT [15], a finding replicated at a regional level in this current study. Using the catheter clinically advocated for a 4.0 ETT (8 FG), CS demonstrated no benefit over OS in preserving regional EELV. Large suction catheters, by obstructing more of the ETT lumen, generate high negative pressures, irrespective of suction method [32–35]. The influence of suction catheter size during CS, but not OS, may also explain the variable results of studies comparing the effect of OS and CS on global EELV, oxygenation and heart rate in newborn infants [10–12, 36]. More recently significant regional loss of EELV and V T has also been reported during CS in preterm infants [23, 37].
The restoration of tidal ventilation and lung volume after ETT suction is as important as the loss during suction. Advocates of CS have argued that lung volume recovers more quickly than following OS [11], possibly accounting for the oxygenation benefits of CS in preterm infants reported in some studies [12]. Our results question this, as recovery of lung volume and ventilation after ETT suction was rapid and uniform throughout the lung. Whilst the ΔZ VT results suggested greater inhomogeneity of ventilation after OS compared to CS, these differences were not significant. Indeed, despite the 10–14-fold greater loss of EELV seen during OS, and CS with an 8 FG catheter compared to 5 FG CS, the regional EELV and V T 10 s after suction did not differ. This rapid recovery is consistent with studies comparing global ΔEELV during and after suction using open and closed methods [10, 13, 15].
Active lung volume recruitment manoeuvres have been advocated after suction, especially OS, and are known to improve recovery of oxygenation in human and animal adult studies [30, 38–40]. Our findings do not support the universal application of active lung volume recruitment after suction during time-cycled pressure-limited ventilation [10, 13, 15, 41]. In many of the suction episode recordings, there was a minimal difference in pre- and post-suction regional EELV and V T using inflating pressures of approximately 20 cmH2O and PEEP. Following these suction episodes, active lung volume recruitment would not be necessary, and is potentially harmful. During some suction episodes, however, large regional EELV and V T differences persisted after suction, suggesting volume inhomogeneity and thus greater potential for lung injury [8, 9]. In these circumstances, active post-suction lung recruitment may well be of benefit. In each animal, suction method and catheter size were not able to predict the resultant distribution of ventilation and volume within the lung after suction, and thus the need for a recruitment manoeuvre. Currently clinicians have no method of determining such volume states as both atelectasis and overdistension cause common bedside parameters, such as oxygenation, to deteriorate. EIT may offer potential as a tool for monitoring post-suction ventilation, and determining an individual’s need for and response to active recruitment after ETT suction.
The marginal benefits of CS demonstrated in our study illustrate the need to consider more than just the volume loss that occurs during ETT suction. As the purpose of ETT suction is to remove secretions, the use of a small CS catheter to preserve ventilation may not be beneficial. Little is known of the relationship between secretion removal and catheter size [34]. However, OS has been shown to remove secretions more effectively than CS in animal models [42] and in vitro [17]. We did not address secretion recovery in the present study, and further investigation of this topic in a purposefully designed study is warranted.
This study has a number of important limitations not previously discussed. The study was performed on animals receiving a muscle relaxant. This inhibits the patient’s efforts to maintain lung volume and may alter regional differences in lung volume and ventilation. We have previously shown that the differences in global EELV between OS and CS are minimal in spontaneously breathing mechanically ventilated infants [10]. Deoxygenation and bradycardia are well described complications of ETT suction in infants [10, 12] and children [11]. We did not report the cardiorespiratory changes during ETT suction as the results in an animal study are unlikely to aid clinical management or add to the existing human data [36]. Airway flow conditions within the trachea, which may influence the volume loss during suction, were not measured. In a previous study turbulent flow was generated using a suction pressure of −140 mmHg with both 5 and 8 FG catheters and a 4.0 ETT [33]. We also elected to describe the EIT data within two lung regions. This simplifies interpretation but may not account for the complexities of gas and tissue distribution within the diseased lung [27]. The smaller chest wall diameter, and thus lesser superimposed pressure [16], may explain the lack of gravity-dependent regional differences we found compared to similar studies performed in adult animal [17] and paediatric studies [14]. We contend that our model is more representative of the diseased neonatal lung with regard to chest wall size and illness severity. Finally, it is possible that the observed regional changes in EELV and V T during and after suction may have been different if the lung was being ventilated on the deflation limb of the pressure-volume relationship [1, 2, 4]. The role of the volume state of the lung before derecruitment requires further investigation.
Conclusion
ETT suction using current clinical methods and catheter sizes causes significant, albeit transient, loss of end-expiratory lung volume and tidal ventilation within all lung regions. Suction catheter size exerted a greater influence on lung volume loss than suction method. Irrespective of suction method and catheter size, recovery of regional lung volume and tidal ventilation after suction was rapid and uniform in this animal model. Future studies of ETT suction practices should consider the regional behaviour of the lung and not be limited to suction method alone.
References
Rimensberger PC, Pache JC, McKerlie C, Frndova H, Cox PN (2000) Lung recruitment and lung volume maintenance: a strategy for improving oxygenation and preventing lung injury during both conventional mechanical ventilation and high-frequency oscillation. Intensive Care Med 26:745–755
Tingay DG, Mills JF, Morley CJ, Pellicano A, Dargaville PA (2006) The deflation limb of the pressure–volume relationship in infants during high-frequency ventilation. Am J Respir Crit Care Med 173:414–420
Gothberg S, Parker TA, Griebel J, Abman SH, Kinsella JP (2001) Lung volume recruitment in lambs during high-frequency oscillatory ventilation using respiratory inductive plethysmography. Pediatr Res 49:38–44
De Jaegere A, van Veenendaal MB, Michiels A, van Kaam AH (2006) Lung recruitment using oxygenation during open lung high-frequency ventilation in preterm infants. Am J Respir Crit Care Med 174:639–645
Rimensberger PC, Pristine G, Mullen BM, Cox PN, Slutsky AS (1999) Lung recruitment during small tidal volume ventilation allows minimal positive end-expiratory pressure without augmenting lung injury. Crit Care Med 27:1940–1945
van Kaam AH, De Jaegere A, Haitsma JJ, van Aalderen WM, Kok JH, Lachmann B (2003) Positive pressure ventilation with the open lung concept optimizes gas exchange and reduces ventilator-induced lung injury in newborn piglets. Pediatr Res 53:245–253
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 18:319–321
Halter JM, Steinberg JM, Schiller HJ, Dasilva M, Gatto LA, Landas S, Nieman GF (2003) Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 167:1620–1626
Schiller HJ, Steinberg J, Halter J, McCann U, Dasilva M, Gatto LA, Carney D, Nieman G (2003) Alveolar inflation during generation of a quasi-static pressure/volume curve in the acutely injured lung. Crit Care Med 31:1126–1133
Hoellering AB, Copnell B, Dargaville PA, Mills JF, Morley CJ, Tingay DG (2008) Lung volume and cardiorespiratory changes during open and closed endotracheal suction in ventilated newborn infants. Arch Dis Child Fetal Neonatal Ed 93:F436–F441
Choong K, Chatrkaw P, Frndova H, Cox PN (2003) Comparison of loss in lung volume with open versus in-line catheter endotracheal suctioning. Pediatr Crit Care Med 4:69–73
Kalyn A, Blatz S, Sandra F, Paes B, Bautista C (2003) Closed suctioning of intubated neonates maintains better physiologic stability: a randomized trial. J Perinatol 23:218–222
Tingay DG, Copnell B, Mills JF, Morley CJ, Dargaville PA (2007) Effects of open endotracheal suction on lung volume in infants receiving HFOV. Intensive Care Med 33:689–693
Wolf GK, Grychtol B, Frerichs I, van Genderingen HR, Zurakowski D, Thompson JE, Arnold JH (2007) Regional lung volume changes in children with acute respiratory distress syndrome during a derecruitment maneuver. Crit Care Med 35:1972–1978
Copnell B, Dargaville PA, Ryan EM, Kiraly NJ, Chin LO, Mills JF, Tingay DG (2009) The effect of suction method, catheter size and suction pressure on lung volume changes during endotracheal suction in piglets. Pediatr Res 64:405–410
Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ (2001) Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164:122–130
Lindgren S, Odenstedt H, Olegard C, Sondergaard S, Lundin S, Stenqvist O (2007) Regional lung derecruitment after endotracheal suction during volume- or pressure-controlled ventilation: a study using electric impedance tomography. Intensive Care Med 33:172–180
Grant CA, Fraser JF, Dunster KR, Schibler A (2009) The assessment of regional lung mechanics with electrical impedance tomography: a pilot study during recruitment manoeuvres. Intensive Care Med 35:166–170
Frerichs I, Braun P, Dudykevych T, Hahn G, Genee D, Hellige G (2004) Distribution of ventilation in young and elderly adults determined by electrical impedance tomography. Respir Physiol Neurobiol 143:63–75
Frerichs I, Dargaville PA, van GH, Morel DR, Rimensberger PC (2006) Lung volume recruitment after surfactant administration modifies spatial distribution of ventilation. Am J Respir Crit Care Med 174:772–779
Dunlop S, Hough J, Riedel T, Fraser JF, Dunster K, Schibler A (2006) Electrical impedance tomography in extremely prematurely born infants and during high frequency oscillatory ventilation analyzed in the frequency domain. Physiol Meas 27:1151–1165
Wrigge H, Zinserling J, Muders T, Varelmann D, Gunther U, von der GC, Magnusson A, Hedenstierna G, Putensen C (2008) Electrical impedance tomography compared with thoracic computed tomography during a slow inflation maneuver in experimental models of lung injury. Crit Care Med 36:903–909
Tingay DG, Armstrong RA, Carlisle HR, Argus B, Davis PG (2009) Closed endotracheal tube suction causes lung volume derecruitment and less homogeneous ventilation in preterm infants. J Paediatr Child Health 45:A34 (abstract)
Barber DC (1989) A sensitivity method for electrical impedance tomography. Clin Phys Physiol Meas 10:368–371
Barber DC (1989) A review of image reconstruction techniques for electrical impedance tomography. Med Phys 16:162–169
Morrow BM, Argent AC (2008) A comprehensive review of pediatric endotracheal suctioning: Effects, indications, and clinical practice. Pediatr Crit Care Med 9:465–477
Albaiceta GM, Taboada F, Parra D, Luyando LH, Calvo J, Menendez R, Otero J (2004) Tomographic study of the inflection points of the pressure–volume curve in acute lung injury. Am J Respir Crit Care Med 170:1066–1072
Meier T, Luepschen H, Karsten J, Leibecke T, Grossherr M, Gehring H, Leonhardt S (2008) Assessment of regional lung recruitment and derecruitment during a PEEP trial based on electrical impedance tomography. Intensive Care Med 34:543–550
Victorino JA, Borges JB, Okamoto VN, Matos GF, Tucci MR, Caramez MP, Tanaka H, Sipmann FS, Santos DC, Barbas CS, Carvalho CR, Amato MB (2004) Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med 169:791–800
Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, Richard JC, Mancebo J, Lemaire F, Brochard L (2003) Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med 167:1215–1224
Cereda M, Villa F, Colombo E, Greco G, Nacoti M, Pesenti A (2001) Closed system endotracheal suctioning maintains lung volume during volume-controlled mechanical ventilation. Intensive Care Med 27:648–654
Kiraly NJ, Tingay DG, Mills JF, Morley CJ, Dargaville PA, Copnell B (2009) The effects of closed endotracheal suction on ventilation during conventional and high frequency oscillatory ventilation. Pediatr Res 66:400–404
Kiraly NJ, Tingay DG, Mills JF, Morley CJ, Copnell B (2008) Negative tracheal pressure during neonatal endotracheal suction. Pediatr Res 64:29–33
Morrow BM, Futter MJ, Argent AC (2004) Endotracheal suctioning: from principles to practice. Intensive Care Med 30:1167–1174
Monaco FJ, Meredith KS (1992) A bench test evaluation of a neonatal closed tracheal suction system. Pediatr Pulmonol 13:121–123
Woodgate PG, Flenady V (2001) Tracheal suctioning without disconnection in intubated ventilated neonates. Cochrane Database Syst Rev, issue 2, art. no. CD003065. doi: 10.1002/14651858.CD003065
van Veenendaal MB, Miedema M, de Jongh FHC, van der Lee JH, Frerichs I, van Kaam AH (2009) Effect of closed endotracheal suction in high-frequency ventilated premature infants measured with electrical impedance tomography. Intensive Care Med 35:2130–2134
Lasocki S, Lu Q, Sartorius A, Fouillat D, Remerand F, Rouby JJ (2006) Open and closed-circuit endotracheal suctioning in acute lung injury: efficiency and effects on gas exchange. Anesthesiology 104:39–47
Almgren B, Wickerts CJ, Hogman M (2004) Post-suction recruitment manoeuvre restores lung function in healthy, anaesthetized pigs. Anaesth Intensive Care 32:339–345
Dyhr T, Bonde J, Larsson A (2003) Lung recruitment manoeuvres are effective in regaining lung volume and oxygenation after open endotracheal suctioning in acute respiratory distress syndrome. Crit Care 7:55–62
Morrow B, Futter M, Argent A (2007) A recruitment manoeuvre performed after endotracheal suction does not increase dynamic compliance in ventilated paediatric patients: a randomised controlled trial. Aust J Physiother 53:163–169
Copnell B, Tingay DG, Kiraly NJ, Sourial M, Gordon MJ, Mills JF, Morley CJ, Dargaville PA (2007) A comparison of the effectiveness of open and closed endotracheal suction. Intensive Care Med 33:1655–1662
Acknowledgments
We thank Magdy Sourial, Shane Osterfield, Scott Dunlop and Ethel Ryan for their assistance in the completion of this project. DGT is supported by a National Health and Medical Research Council Clinical Research Fellowship (grant ID 491286).
Conflict of interest statement
The authors declare that there are no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Functional EIT movie of closed ETT suction with the Ballard Trachcare™ inline suction system using firstly a 5 FG and then an 8 FG suction catheter. (MP4 6.67 mb)
Rights and permissions
About this article
Cite this article
Tingay, D.G., Copnell, B., Grant, C.A. et al. The effect of endotracheal suction on regional tidal ventilation and end-expiratory lung volume. Intensive Care Med 36, 888–896 (2010). https://doi.org/10.1007/s00134-010-1849-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1849-x