Abstract
Purpose
To assess the relationship between early daily fluid balance (FB) and 90-day outcome in adult patients treated with extracorporeal membrane oxygenation (ECMO).
Design
Retrospective observational study.
Setting
Tertiary referral centre for ECMO.
Patients
115 patients treated with ECMO for refractory heart failure and 57 patients treated with ECMO for refractory respiratory failure.
Methods
We analysed the association between early daily FB versus hospital and 90-day mortality using multivariable logistic regression model, Cox proportional-hazards model and propensity score.
Results
We obtained detailed demographic, clinical, and biochemical data, daily FB, and continuous renal replacement days. Fifty-seven per cent of patients had acute kidney injury (AKI) at ECMO initiation, and 60 % (n = 103) of patients received continuous renal replacement therapy (CRRT) during ECMO course, beginning at a median of 1 (0–3.5) days after ECMO initiation. Overall 90-day mortality was 24 %. Survivors exhibited lower daily FB from day 3 to day 5. After adjustments, Acute Physiology and Chronic Health Evaluation (APACHE) III, CRRT during the first 3 days, major bleeding event at day 1 and positive FB on day 3 were independent predictors of 90-day mortality. Positive FB at ECMO day 3 remained an independent predictor of hospital and 90-day mortality, regardless of the statistical model used or the inclusion of a propensity score to have positive FB.
Conclusions
Positive FB at ECMO day 3 is an independent predictor of 90-day mortality. Further interventional studies aimed at testing the value of strategy of tight control of FB during the early ECMO period are now warranted.
Similar content being viewed by others
Introduction
Extracorporeal membrane oxygenation (ECMO) is considered an effective rescue therapy for severe acute lung or cardiac disease [1, 2]. Large-volume intravenous fluid infusions are often required after ECMO initiation [1, 3] due to haemorrhage [4–7] or to minimize venous access insufficiency and target an appropriate ECMO flow [1, 3]. Despite increasing experience [8–10] and major technical improvements [11], major fluid overload remains a common characteristic in ECMO patients [12]. In addition, acute kidney injury (AKI) with need for renal replacement therapy (RRT) is one of the most frequent additional organ failures, occurring in ~50 % of ECMO patients [4–7]. AKI is known to be associated with negative outcomes [13, 14] in patients requiring ECMO and may also predispose towards development of positive fluid balance (FB) [15–19]. Although fluid overload is common in ECMO patients [12] and the possible adverse effects of positive FB on mortality are widely recognized [15–19], the specific impact of fluid overload on outcome in adult ECMO patients has never been investigated. Moreover, FB status during ECMO is a potentially modifiable factor, while if optimally managed may improve patient outcomes.
The aim of this investigation is to describe the relationship between early FB status (within the first 3 days of ECMO initiation) and outcome within an adult population treated with ECMO and to account for confounding factors such as AKI or RRT use.
Patients and methods
Setting
This study was conducted in a 45-bed medical–surgical intensive care unit (ICU) of a university hospital in Melbourne, Australia (The Alfred Hospital). This hospital provides heart and lung transplantation services for the States of Victoria, South Australia and Tasmania. The ICU operates an ECMO referral service and retrieves patients on ECMO from the southern states of Australia. The study protocol was in accordance with the ethical standards of our institution’s Committee for the Protection of Human Research Subjects. The need for informed consent was waived by the Human Research Ethics Committee (number 324-13).
Patients
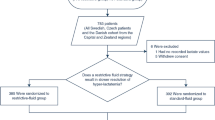
We retrospectively analysed the charts of consecutive patients who received veno-arterial ECMO (VA-ECMO) or veno-venous ECMO (VV-ECMO) for refractory cardiogenic shock or acute respiratory failure, respectively, in our ICU from 1 March 2006 to 1 March 2013. Only patients who were alive on the third day of ECMO treatment and had available data about FB were eligible for the study. Twenty-one patients who had ECMO for ≤48 h and were not exposed to ECMO long enough to identify fluid-overload-related outcome were excluded from the study. Two patients with insufficient data (ECMO retrievals where FB information was not available from the referring hospital) were excluded from analysis (Fig. 1).
ECMO support
The decision to use ECMO was made by the treating intensive care specialist or cardiac/thoracic surgeon (for perioperative cardiac or lung support) according to local criteria as previously described [20]. ECMO indications and cannulation procedure details are provided in the Electronic Supplementary Material (ESM).
Standard procedure for care of ECMO patients requiring CRRT
The decision to use continuous RRT (CRRT) during ECMO was made by the treating intensive care specialist. Typical indications for CRRT included potassium level >6.5 mmol/L or pH <7.2 or urea concentration >25 mmol/L or creatinine level >300 µmol/L or clinically significant organ oedema (e.g. pulmonary oedema). CRRT (Prismaflex™; Gambro, Lund, Sweden) was performed via the ECMO circuit in all patients. Blood entered the CRRT machine immediately distal to the pump-head and was returned proximal to the oxygenator [21]. For patients with no active bleeding, anticoagulation was provided by the ECMO circuit to target an activated partial thromboplastin time (APTT) of 50–70 s. The anticoagulant strategy was the same whether CRRT was required or not. After ECMO removal, a dialysis central catheter was inserted if CRRT was still needed. The CRRT filter was routinely changed after 72 h of continuous use (manufacturer recommendation) or earlier due to clotting.
Administration of fluid was not protocolised but was decided by the treating intensivist. Crystalloid solutions were used for maintenance fluid. The choice of fluid bolus for resuscitation included crystalloid or 4 % albumin. Gelatin and hydroxyethyl starch solutions were not used. Access pressures were not routinely monitored, and fluid was given when suction events occurred with associated reduction in ECMO blood flow. Recurrent episodes of suction led to repositioning or addition of another access cannula or sustained reduction in revs. The minimum haemoglobin target was 8.0 g/dL [22]. Coagulation tests including haemolysis screening (plasma free haemoglobin) were performed at least daily. ECMO circuit changes were performed if there was clinically significant haemolysis or fibrinolysis, with or without clot within the oxygenator. Bed-side care was delivered by trained ICU nursing staff with patient-to-nurse ratio of 1:1 under supervision of ECMO-trained intensivists.
Data collection
The following data were extracted from patient records and ICU databases: age, sex, body mass index, underlying medical conditions, Acute Physiology and Chronic Health Evaluation (APACHE) III score [23], Sequential Organ Failure Assessment (SOFA) [24], immunosuppression status, reason for ECMO initiation, days in ICU and hospital before ECMO initiation, type of ECMO (VA versus VV) and whether the patient was retrieved from another hospital on ECMO. A SOFA score with the renal component removed was calculated to account for severity of illness distinct from that caused by AKI. Immunosuppression and chronic renal insufficiency were defined according to APACHE II criteria [25]. Daily data from ECMO initiation to ICU discharge (or day 28) included daily FB, urine output, CRRT use, CRRT circuit change, furosemide dose, ECMO circuit change, major bleeding events, and haematological and renal functional variables (haemoglobin, plasma free haemoglobin, urea and creatinine before CRRT).
Daily FB was calculated as the difference between fluid administered (intravenous fluids, blood products, enteral fluids, RRT replacement fluids) and fluid lost (dialysis effluent–dialysate from CRRT, urine output, enteral losses, drain losses) in a 24-h period. Negative daily FB was present when fluid loss was greater than fluid administered per day [26]. Major bleeding event was defined as bleeding which led to a haemostatic treatment (pleural drainage, surgical procedure, arterial embolization, gastrointestinal endoscopy), transfusion of ≥5 blood units of packed red blood cells during 24 h, in cases of intracerebral haemorrhage or death.
To determine AKI severity at ECMO initiation, patients were assigned to risk, injury, failure, loss and end-stage kidney disease (RIFLE) categories on the basis of serum creatinine and urine output [27]. Patients were classified into no-AKI, RIFLE-Risk, RIFLE-Injury or RIFLE-Failure groups at ECMO initiation [27]. Patients already on CRRT at ECMO initiation were classified into the RIFLE-Failure group. Patients with AKI were defined as RIFLE-Risk, RIFLE-Injury and RIFLE-Failure groups. The relationship between FB and outcomes was examined by considering FB as a continuous variable and after dichotomizing into two groups on the basis of daily FB (negative and positive daily FB). Additional sensitivity analyses were performed in the following subgroups: patients who required or not CRRT, acute respiratory distress syndrome (ARDS) diagnosis, cardiogenic shock diagnosis, and AKI and no-AKI groups.
The primary outcome considered was mortality at 90 days after 3 days on ECMO. Secondary outcomes included hospital mortality, time to death from ECMO day 3 up to day 90 and time to in-hospital death. Mechanical-ventilation-free days at day 60, duration of mechanical ventilation, ECMO-free days at day 28, duration of ECMO run, duration of CRRT, and ICU and hospital length of stay (LOS) were also assessed.
Statistical analyses
Data were initially assessed for normality. Continuous normally distributed variables were compared using Student t tests or analysis of variance (ANOVA) and are presented as mean (standard deviation), whilst non-normally distributed variables were compared using Wilcoxon rank-sum tests or Kruskal–Wallis tests and are presented as median (interquartile range, IQR). Categorical variables were compared using chi-square test for equal proportion or Fisher’s exact tests and are reported as number (percentage). For pairwise comparisons of RIFLE categories and fluid balance over time, Bonferroni adjustment for multiple comparisons was employed.
Multivariate logistic regression was used to identify early independent risk factors for 90-day mortality in ECMO patients, with results presented as odds ratio (95 % confidence interval, CI). Multivariate models were constructed using both stepwise selection and backwards elimination techniques before undergoing a final assessment for clinical and biological plausibility. With the exception of primary cause of ECMO and year (automatically forced into the model), all other variables before day 3 with univariate p < 0.10 were considered for model inclusion (i.e. SOFA score at ECMO cannulation, APACHE III, CRRT during the first 3 days, daily FB, urine output, and bleeding events at day 1 and day 3 on ECMO). Analysis of time to death (censored at 90 days after 3 days on ECMO) was compared using Gehan–Breslow–Wilcoxon test and is presented using Kaplan–Meier curves. The optimal threshold of FB at ECMO day 3 for mortality was calculated by the Youden index.
To test the robustness of any association between mortality and early positive FB, interactions with other covariates (i.e. primary diagnosis ARDS, RRT from day 1 to day 3, major bleeding at day 1, APACHE III) were tested. Additional models were applied to data analysis. These models included time-dependent modelling and Cox proportional-hazards modelling. Such models included a propensity score. The propensity score was estimated by using a multivariate logistic regression of each patient receiving positive FB at ECMO day 3 or not. The model included all baseline characteristic variables and collected data before ECMO day 3 using a stepwise selection procedure with inclusion criterion of p < 0.2. Variables retained in the propensity model to have positive FB at day 3 are displayed in Table ESM-1. This propensity score was included as covariate in the models on 4 outcomes: hospital death, death at day 90, time to hospital death and time to death at day 90, respectively. Multivariate analyses to identify independent factors associated with death at day 90 were re-run in 4 subgroups: AKI at ECMO initiation, CRRT during the first 3 days, ARDS and cardiogenic shock. All analyses were run twice with FB at ECMO day 3 considered either as a binomial value (positive versus negative FB) or as a continuous value.
Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA), and a two-sided p value of 0.05 was considered to be statistically significant.
Results
Study population
During the 7-year study period, 172 patients (44 ± 15 years old, 66 % male) fulfilled our inclusion criteria (Fig. 1). Of these, 115 received ECMO for primarily cardiac causes, whereas 57 had ECMO for acute respiratory failure (53 patients for ARDS, 4 patients for end-stage respiratory failure). Median ICU LOS was 18 (12–30) and median hospital LOS was 35 (21–59) days. Overall 90-day mortality was 24 %. Mortality in the cardiogenic shock group and in the acute respiratory failure group was 23 and 25 %, respectively. Overall 90-day mortality was higher for patients who required CRRT (p < 0.0006, Table 2). The main characteristics of the whole population at ECMO initiation are presented in Table 1 and Table ESM-2.
Daily fluid balance in the study population
Figure ESM-1 depicts the evolution of FB management during the first 3 days on ECMO over the study period. It is worth noting that the mean FB at day 3 significantly decreased between 2006 and 2013. However, “year” was not retained as an independent risk factor of mortality. A difference in daily FB between survivors and non-survivors was detected on the third day of ECMO and persisted until day 5 (Fig. 2). On day 1 and day 2, both groups had a similar positive FB. On the following days (i.e. from day 3 to day 5), survivors exhibited significantly lower FB (Fig. 2). Table 2 presents main characteristics and outcomes according to FB at ECMO day 3. Briefly, the number of patients with ARDS or cardiogenic shock was similar according to FB status at day 3. Similarly, same number received RRT during their ICU stay. In addition, compared with those with negative FB, patients with positive FB exhibited fewer mechanical-ventilation-free days at day 60 and higher day-90 mortality [44 (16–54) versus 37 (0–48) days, p = 0.03 and 14 versus 31 %, p = 0.009, respectively]. Survival plots were used to compare positive versus negative FB at day 3. Patients with positive FB had significantly lower survival than those with negative FB (i.e. 70 versus 85 % at day 90) (Fig. ESM-2). In addition, positive FB of 1–2 L/day at ECMO day 3 was the optimal threshold for mortality, with a mean sensitivity and a specificity of 44 and 82 %, respectively (Fig. ESM-3; Table ESM-3).
Multivariate and adjusted analyses
After adjusting for other significant confounding variables (APACHE III score, CRRT during the first 3 days, major bleeding at day 1) using logistic regression analysis, an increasingly positive FB on day 3 was an independent predictor of death at day 90 (Table 3). After adjusting for the propensity to have positive FB through the inclusion of a propensity score and considering FB as a binomial or a continuous value, positive FB at day 3 remained an independent predictor of 90-day mortality (Table 3). Similarly, positive FB at day 3 was also independently associated with time to death at day 90, in-hospital mortality and time to hospital death using Cox proportional-hazards modelling.
No significant interactions between positive FB at day 3 and APACHE III score, CRRT or major bleeding during the early ECMO period were found.
Acute kidney injury, continuous renal replacement therapy, primary diagnosis and daily fluid balance
Ninety-eight patients (57 %) exhibited AKI at ECMO initiation. Comparison between characteristics and outcome according to RIFLE categories at ECMO initiation is presented in Tables ESM-4 and ESM-5. Briefly, RIFLE categories at ECMO initiation were distributed as follows: no-AKI [n = 74 (43 %)], RIFLE-Risk [n = 23 (13 %)], RIFLE-Injury [n = 39 (23 %)], RIFLE-Failure [n = 36 (21 %)]. Greater RIFLE severity at ECMO initiation carried a greater 90-day mortality rate. Such mortality ranged from 15 % in the no-AKI group to 47 % in the RIFLE-Failure group (Table ESM-5). Compared with no-AKI patients, mortality at day 90 of patients with AKI was higher (31 versus 15 %, p < 0.001). However, positive FB at day 3 was still independently associated with death at day 90 in both AKI and no-AKI groups (Tables ESM-6 and ESM-7).
Baseline characteristics and outcome according to CRRT during the first 3 ECMO days are presented in Table ESM-8. Briefly, 72 (42 %) patients received CRRT during the first 3 days after ECMO initiation. Primary reasons for CRRT were acute renal failure (76 %), fluid overload (21 %) and electrolyte imbalance (3 %). Thirty-eight patients (35 %) died at day 90. Consistently, FB at day 3 was still higher in non-survivors (p < 0.05; Fig. ESM-4). In addition, positive FB at ECMO day 3 was independently associated with 90-day mortality within patients with CRRT (Tables ESM-9 and ESM-10).
Similarly, positive FB at day 3 was still an independent factor associated with 90-day mortality in patients with ARDS or with cardiogenic shock (Tables ESM-11 and ESM-12).
Discussion
Key findings
In an adult population requiring ECMO, we found that, even after adjustments for propensity score and all illness severity markers, positive FB at day 3 is a robust independent predictor of 90-day mortality. This significant difference in FB between 90-day survivors and non-survivors was noted from day 3 to day 5 on ECMO. In addition, we confirmed a trend to increased 90-day mortality with more severe RIFLE scores with an overall 35 % 90-day mortality for patients treated with CRRT. In addition, CRRT during the first 3 days on ECMO was also an independent risk factor of 90-day mortality.
Comparison with previous studies
Despite the overall lower than expected 90-day mortality rate in our cohort [13, 28–30], we confirm that positive FB at day 3 and CRRT during the first 3 days on ECMO are independent risk factors for mortality [13, 28, 30, 31]. In the past decade, the view that liberal fluid administration preserves renal function has been challenged [32–35]. A number of studies, in different settings, have stressed the negative impact of fluid overload on mortality, oxygenation, and duration of mechanical ventilation and ICU LOS [16, 26, 36–40]. In a retrospective analysis of 1,400 patients enrolled in the RENAL study [41], negative daily FB was associated with decreased risk of death at 90 days, even after adjustments for propensity score and all illness severity markers [26]. ECMO patients are especially exposed to fluid overload, and FB management is a common issue in this population [42–44]. Selewski et al. [44], in a retrospective study of 57 ECMO children requiring CRRT, recently addressed the association between mortality and fluid overload at RRT initiation, fluid removal during CRRT and the kinetics of fluid removal. Median fluid overload at CRRT initiation and CRRT discontinuation were significantly lower in survivors. However, after adjusting for confounders, fluid overload at CRRT discontinuation was not significantly associated with mortality, highlighting the fact that fluid status early in the patient’s course is most predictive of survival. The addition of in-line continuous haemofiltration to the ECMO circuit (which avoids the need for a separate central venous access line) has been suggested as a potential solution for improved fluid homeostasis in concert with native diuresis [45]. In 15 newborn patients matched with 46 controls, such addition of CRRT to the ECMO circuit improved outcomes by reducing time on ECMO and on ventilation as well as improving fluid balance management [43]. Similarly, animal models of ECMO have suggested there may be a direct protective effect from CRRT with reduced inflammatory markers, improvement of mean perfusion pressure, systemic vascular resistance and oxygen delivery [46]. However, CRRT might increase the risk of ECMO oxygenator failure. In our series, the number of ECMO circuits used was significantly higher in the CRRT group (Table ESM-8), a finding which has been reported by others as associated with worse outcome [47]. In addition, we did not find any independent association between AKI and positive FB at day 3. However, patients were classified at ECMO initiation, and some of the 74 no-AKI patients could have developed AKI thereafter.
Contribution to knowledge
Our study provides findings of an independent association between early positive FB and 90-day mortality in ECMO patients regardless of the reason for ECMO. Although fluid overload may reflect only the illness severity of these patients [12], these results raise the possibility that early control of FB on ECMO may be beneficial. However, ECMO may also intrinsically predispose to fluid overload. Recurrent suction episodes due to access insufficiency may both predispose to increased fluid administration and represent inadequate ECMO support which in turn manifests as adverse mortality outcomes. The ECMO centrifugal pumps are preload dependent, and intravenous fluids are, especially in the first 48 h after ECMO initiation, frequently administered to target appropriate ECMO flow [1, 3]. In fact, the positive FB seen during the first two days on ECMO did not show an association with 90-day mortality (Table 2; Fig. 2). As demonstrated with acute lung injury with sepsis [48], further studies are warranted to study the outcome of a strategy combining early aggressive fluid resuscitation associated with later fluid restriction in ECMO patients.
Implications of study findings
The consistent association between positive FB at day 3 and mortality after multiple adjustments may suggest the need to exert prudence with fluid administration in ECMO patients after the first 48 h. Then, this finding could emphasize the need for proactive strategies to reduce fluid overload as soon as possible. Recent advances in incorporation of CRRT with ECMO devices allow thorough control of fluid balance with lower exposure to diuretics [44]. This study should prompt clinicians and researchers to develop trials to confirm the negative impact of fluid overload in ECMO on outcome and to develop specific strategies for management of AKI and FB. Proactive use of CRRT and diuretics during ECMO, aiming to improve fluid overload as soon as possible, might be investigated.
Strengths and limitations
To the best of our knowledge, this is the first time that an association of positive FB with unfavourable outcome has been reported in a large adult ECMO cohort. Our findings have potential therapeutic implications and may lead to studies comparing a conservative versus liberal fluid therapy approach in these unique patients. Our study, however, has a retrospective design and was performed in a single centre with no standardization of the timing of CRRT during ECMO. In addition, our results depend on local experience, practice and management of ECMO and CRRT. However, we believe other expert centres can reproduce such clinical management. We studied a mixed population of patients with acute refractory cardiogenic shock and acute respiratory failure, who had received VA- or VV-ECMO support. We recognize that subgroups of such patients may behave differently and that this study is underpowered to detect variation between small populations. However, these two different populations had the same mortality rate. Moreover, even after multiple adjustments and propensity score use, the negative impact of FB on mortality was still noted in both ECMO populations. We were not able to obtain accurate FB data before ECMO initiation. However, a high proportion of patients received ECMO during their first day in ICU (115 of 172 patients). Similarly, the dose of vasopressors was not collected. A constant haemodynamics SOFA subscore at 4 in both survivor and non-survivor groups suggests that the epinephrine or norepinephrine dose was greater than 0.1 µg/kg/min at ICU admission and at ECMO initiation. The type of fluids used was not collected. However, hydroxyethyl starch, which has been shown to be associated with a need for RRT [49, 50], was not available during the study period. We cannot exclude that the results of our multivariable analyses might have been biased by residual confounding not accounted for in this study. Finally, although we have shown that positive FB on day 3 of ECMO is associated with adverse outcomes, this study cannot rule out that those associations may simply reflect the extreme illness severity. Whether strategies to limit fluid accumulation either are possible or would change patient outcomes must be determined.
Conclusions
Our findings show that early positive FB, especially at day 3, is a robust independent predictor of 90-day mortality during ECMO, regardless of the primary diagnosis, AKI, or RRT use. In addition, we confirm that AKI during ECMO in adult patients is frequent and that those requiring CRRT are at greater risk of 90-day mortality. Further prospective studies aimed at testing the value of strategy of tight control of FB during the first 5 days of ECMO appear desirable.
References
Brodie D, Bacchetta M (2011) Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 365:1905–1914
Sayer GT, Baker JN, Parks KA (2012) Heart rescue: the role of mechanical circulatory support in the management of severe refractory cardiogenic shock. Curr Opin Crit Care 18:409–416
Combes A, Brechot N, Luyt CE, Schmidt M (2012) What is the niche for extracorporeal membrane oxygenation in severe acute respiratory distress syndrome? Curr Opin Crit Care 18:527–532
Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B (2013) Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 97(2):610–616
Paden ML, Warshaw BL, Heard ML, Fortenberry JD (2011) Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med 12:153–158
Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, Patroniti N, Antonelli M, Pesenti A, Pappalardo F (2013) A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc 15:172–178
Zwiers AJ, de Wildt SN, Hop WC, Dorresteijn EM, Gischler SJ, Tibboel D, Cransberg K (2013) Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14-year cohort study. Crit Care 17:R151
Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettila V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M (2009) Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Nieszkowska A, Dupont H, Ouattara A, Leprince P, Chastre J, Combes A (2013) The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 39:1704–1713
Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, Cosgrove DM 3rd (2001) Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg 122:92–102
MacLaren G, Combes A, Bartlett RH (2012) Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 38:210–220
Maclaren G, Butt W (2009) Controversies in paediatric continuous renal replacement therapy. Intensive Care Med 35:596–602
Lin CY, Chen YC, Tsai FC, Tian YC, Jenq CC, Fang JT, Yang CW (2006) RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant 21:2867–2873
Swaniker F, Kolla S, Moler F, Custer J, Grams R, Barlett R, Hirschl R (2000) Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg 35:197–202
Gillespie RS, Seidel K, Symons JM (2004) Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol 19:1394–1399
Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD (2011) Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 6:966–973
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL (2008) A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12:R74
Rosenberg AL, Dechert RE, Park PK, Bartlett RH (2009) Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med 24:35–46
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325
Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, Scheinkestel C, Pellegrino V (2013) Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care 17:R73
Askenazi DJ, Selewski DT, Paden ML, Cooper DS, Bridges BC, Zappitelli M, Fleming GM (2012) Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 7:1328–1336
Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Brechot N, Merceron S, Luyt CE, Trouillet JL, Chastre J, Leprince P, Combes A (2013) Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 39:838–846
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A et al (1991) The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619–1636
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, Lo S, McArthur C, McGuiness S, Norton R, Myburgh J, Scheinkestel C, Su S (2012) An observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial. Crit Care Med 40:1753–1760
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure––definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Chen YC, Tsai FC, Chang CH, Lin CY, Jenq CC, Juan KC, Hsu HH, Chang MY, Tian YC, Hung CC, Fang JT, Yang CW (2011) Prognosis of patients on extracorporeal membrane oxygenation: the impact of acute kidney injury on mortality. Ann Thorac Surg 91:137–142
Hei F, Lou S, Li J, Yu K, Liu J, Feng Z, Zhao J, Hu S, Xu J, Chang Q, Liu Y, Wang X, Liu P, Long C (2011) Five-year results of 121 consecutive patients treated with extracorporeal membrane oxygenation at Fu Wai Hospital. Artif Organs 35:572–578
Yan X, Jia S, Meng X, Dong P, Jia M, Wan J, Hou X (2010) Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the Acute Kidney Injury Network criteria. Eur J Cardiothorac Surg 37:334–338
Kielstein JT, Heiden AM, Beutel G, Gottlieb J, Wiesner O, Hafer C, Hadem J, Reising A, Haverich A, Kuhn C, Fischer S (2013) Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant 28:86–90
Kellum JA, Cerda J, Kaplan LJ, Nadim MK, Palevsky PM (2008) Fluids for prevention and management of acute kidney injury. Int J Artif Org 31:96–110
Kellum JA, Ronco C, Mehta RL (2008) Fluid management in acute kidney injury. Int J Artif Org 31:94–95
Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, Mehta R (2005) Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care 11:533–536
Prowle JR, Kirwan CJ, Bellomo R (2013) Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 10(1):37–47
Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL (2012) Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med 13:253–258
Fulop T, Pathak MB, Schmidt DW, Lengvarszky Z, Juncos JP, Lebrun CJ, Brar H, Juncos LA (2010) Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. ASAIO J 56:333–337
Sakr Y, Vincent JL, Reinhart K, Groeneveld J, Michalopoulos A, Sprung CL, Artigas A, Ranieri VM (2005) High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest 128:3098–3108
Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous CA (2005) Fluid balance and weaning outcomes. Intensive Care Med 31:1643–1647
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638
Blijdorp K, Cransberg K, Wildschut ED, Gischler SJ, Jan Houmes R, Wolff ED, Tibboel D (2009) Haemofiltration in newborns treated with extracorporeal membrane oxygenation: a case-comparison study. Crit Care 13:R48
Hoover NG, Heard M, Reid C, Wagoner S, Rogers K, Foland J, Paden ML, Fortenberry JD (2008) Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med 34:2241–2247
Selewski DT, Cornell TT, Blatt NB, Han YY, Mottes T, Kommareddi M, Gaies MG, Annich GM, Kershaw DB, Shanley TP, Heung M (2012) Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med 40:2694–2699
Meyer RJ, Brophy PD, Bunchman TE, Annich GM, Maxvold NJ, Mottes TA, Custer JR (2001) Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med 2:238–242
Mu TS, Palmer EG, Batts SG, Lentz-Kapua SL, Uyehara-Lock JH, Uyehara CF (2012) Continuous renal replacement therapy to reduce inflammation in a piglet hemorrhage-reperfusion extracorporeal membrane oxygenation model. Pediatr Res 72:249–255
Smalley N, MacLaren G, Best D, Paul E, Butt W (2012) Outcomes in children with refractory pneumonia supported with extracorporeal membrane oxygenation. Intensive Care Med 38:1001–1007
Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O (2008) Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med 36:1518–1522
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, McGuinness S, Rajbhandari D, Taylor CB, Webb SA (2012) Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 367:1901–1911
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Soe-Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K, Kjaeldgaard AL, Fabritius ML, Mondrup F, Pott FC, Moller TP, Winkel P, Wetterslev J (2012) Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 367:124–134
Acknowledgments
M.S. was supported by the French Intensive Care Society (SRLF), the Fonds de Dotation Recherche en Santé Respiratoire 2012, the Collège des Enseignants de Réanimation Médicale and the Fonds d’Étude et de Recherche du Corps Médical des Hôpitaux de Paris.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message:
Early positive fluid balance, especially at day 3, is a robust independent predictor of 90-day mortality during ECMO, regardless of primary diagnosis, acute kidney injury or renal replacement therapy use.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, M., Bailey, M., Kelly, J. et al. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med 40, 1256–1266 (2014). https://doi.org/10.1007/s00134-014-3360-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3360-2