Abstract
Purpose
To estimate the direct and indirect (contextual) effects of the factorized constituents of selective digestive decontamination and selective oropharyngeal decontamination (SDD/SOD), being topical antibiotic (TA) and protocolized antifungal prophylaxis (PAFP), on ICU-acquired candidemia.
Methods
A broad range of ICU candidemia incidence studies were sourced to serve as points of reference. The candidemia incidence was extracted from component (control and intervention) groups decanted from studies of various designs (concurrent or non-concurrent) and whether investigating SDD/SOD versus non-TA methods of ICU infection prevention. The candidemia incidences were summarized in regression models using generalized estimating equation (GEE) methods. Groups derived from observational studies (no prevention method under study) provided an overarching external benchmark candidemia incidence for calibration.
Results
Within studies investigating SDD/SOD, the mean (and 95 % confidence interval) candidemia incidence among concurrent component groups (40 control; 2.4 %; 1.7–3.2 % and 43 intervention groups; 2.4 %; 1.6–3.1 %), but not non-concurrent control groups (11 groups; 1.6 %; 0.1–2.7 %), is higher than that of the benchmark candidemia incidence derived from 54 observational groups (1.5 %; 1.2–1.9 %). The TA constituent within SDD/SOD has significant direct and indirect (contextual) effects in GEE models even after adjusting for the publication year and the group-wide presence of either candidemia risk factors or PAFP use.
Conclusion
The TA constituent of SDD/SOD is associated with a contextual effect on candidemia incidence which is similar in magnitude to that of the conventional candidemia risk factors and against which PAFP partially attenuates. This increase is inapparent within individual SDD/SOD studies examined in isolation.
Similar content being viewed by others
Introduction
Candidemia is acquired by approximately 1 % of prolonged stay patients in the ICU and is associated with a high attributable mortality [1]. In this patient group, the acquisition of colonization with Candida in the oropharynx and gastrointestinal tract is a key intermediary step toward the development of invasive candidiasis and candidemia [2].
Selective digestive decontamination and selective oropharyngeal decontamination (SDD/SOD) are methods using multiple topical antibiotics (TA) for preventing bacterial colonization and infection in the ICU [3, 4]. Among the range of methods for the prevention of ventilator-associated pneumonia (VAP) and other ICU-acquired infections, the evidence base for SDD/SOD appears most compelling [5–10].
The impact of SDD/SOD on the incidence of Candida infections is of great interest for several reasons. Firstly, antibiotic usage, both in number and duration, is a strong patient-level risk factor for the acquisition of colonization and infection with Candida [11]. Secondly, SDD/SOD commonly includes topical amphotericin as protocolized antifungal prophylaxis (PAFP) to control fungal overgrowth. While SDD/SOD appears to be effective at reducing the incidence of fungal colonization and invasive fungal infections [9, 10], the impact of SDD/SOD on candidemia is unclear with conflicting data [4, 9, 10]. Thirdly, the possibility that SDD/SOD may have an indirect (contextual) effect on ICU-acquired infection and that a non-concurrent study was required to estimate its effect size had been postulated in the first study of SDD/SOD [3]. Several observations suggest that the TA component of SDD/SOD creates a profound contextual influence on concurrent patients within an ICU which is inapparent at the level of any single trial [12, 13]. Herd protection resulting from vaccination undertaken within a population is an example of a contextual effect. In this respect, the magnitude of this contextual effect on bacteremia [14] and pneumonia [15] incidences may be both stronger and contrary in direction to that of any direct effect of the SDD/SOD under study. Hence, the contextual effect of TA on the incidence of candidemia within the studies of SDD/SOD warrants closer scrutiny. This estimation requires a calibration of the candidemia incidence within groups of SDD/SOD studies versus from studies of similar patient groups within the broader literature.
This meta-analysis recently appeared in part as a poster presentation [16].
Materials and methods
Study selection and decanting of groups
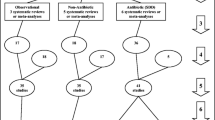
The literature search and analytic approach used here follow seven steps (Fig. 1; numbered arrows):
Flow chart of literature search, study and group decant and analysis plan. Search method and classification of eligible studies and subsequent decanting of component groups. The seven numbered arrows to the right represent steps in the process as discussed in the methods; steps 1–5 refer to studies and steps 6–7 refer to component groups decanted from the studies being control (rectangles) and intervention (ovals) groups from ICU-based studies of infection prevention methods. Cohorts of ICU patients without a prevention method under study are observation groups (diamond) from which the benchmark is derived and against which the component groups were calibrated. The horizontal (blue) dotted rectangles at step 7 represent the strata of control and intervention component groups. Note, the total numbers do not tally as some systematic reviews and studies each provided studies and groups in more than one category, respectively
-
1.
An electronic search of PubMed, The Cochrane database, and Google Scholar for systematic reviews containing potentially eligible studies was undertaken using the following search terms: “ventilator associated pneumonia”, “mechanical ventilation”, “intensive care unit”, “blood stream infection”, candidemia, fungal infection, “bacteremia”, each combined with either “meta-analysis” or “systematic review” up to December 2013.
-
2.
Systematic reviews of studies of patient populations requiring prolonged (more than 24 h) ICU admission, regardless of how prolonged had been defined, were then streamed into one of three categories: systematic reviews containing studies in which there was no study intervention for preventing ICU infections, studies with an intervention that was not TA, and studies of SDD/SOD. For the purpose of this study, SDD/SOD is defined here as the application of topical antibiotic (TA) prophylaxis to the oropharyngeal route without regard to the following: the specific TA constituents, concomitant gastric applications of TA, or the protocolized prophylaxis with either an antifungal or a parenteral antibiotic.
-
3.
The studies were screened against the following eligibility criteria. Inclusion criteria: either candidemia or bacteremia incidence data extractable as an incidence proportion expressed using as the denominator the number of patients with a prolonged ICU stay. Exclusion criteria: studies limited to patients with the acute respiratory distress syndrome. Studies in a language other than English were included when the required data had been abstracted in an English-language systematic review.
-
4.
A hand search was undertaken for additional studies meeting the eligibility criteria.
-
5.
All eligible studies were then collated and any duplicate studies were removed.
-
6.
Groups of prolonged stay patient populations from studies without an infection prevention method under study were labelled as observational groups. The studies of infection prevention were classified as follows. Among the non-TA-based methods of infection prevention are studies with a broad range of interventions. The SDD/SOD studies were further subclassified as to whether the control group was concurrent and co-located within the same ICU as the intervention group (concurrent control) or not (non-concurrent).
-
7.
The component groups of the intervention studies were decanted into strata of control groups and intervention groups. In this analysis, the exposure of interest is TA. Hence, among the studies of SDD/SOD, all groups that had received TA were classified as an intervention group and all groups that did not were classified as a control group regardless of how the group had been classified in the original study and whether or not PAFP was used.
Data extraction, display, and analysis
The candidemia incidence per 100 patients was extracted for each group from those studies which declared this data. However, being a rare (less than 2 %) end point, a non-report from any group could be construed as a zero event rate which would provide evidence against a contextual effect. On this basis the studies of SDD/SOD were scrutinized for any mention of the word fungal or Candida anywhere in the text or tables whether or not the candidemia incidence proportion was declared. Those SDD/SOD studies with a bacteremia incidence but for which the candidemia incidence was not declared were identified with a presumption (imputation) that the candidemia incidence for each of these groups was zero (the base model).
Studies were identified that restricted patient inclusion to only those at high risk for candidemia on the basis of one or more of the following risk factors: use of total parenteral nutrition (TPN), major gastrointestinal surgery or gastrointestinal perforation, mechanical ventilation for longer than 7 days, colonization with candida (however this had been defined), acute pancreatitis, and liver transplantation. The group-wide use of either PAFP by any agent or route or topical placebo was identified.
Three methods were used to display and analyze the candidemia proportion data: scatter plots, caterpillar plots, and multi-level regression models using generalized estimating equation (GEE) methods [17–20]. The justification for the three methods and the details of their execution are described in the Electronic Supplementary Material (ESM) as well as previously [14].
Results
There were 103 studies of which 36 were sourced from eight systematic reviews [5–10, 21, 22]. A total of 198 groups were decanted from these 103 studies with 54 groups from observational studies (ESM Table 1; see Electronic Supplementary Material for additional ESM diagram, ESM tables, ESM figures, and ESM references), 36 groups from studies of various non-TA methods of infection prevention (ESM Table 2), and 108 groups from studies of SDD/SOD that had used either non-concurrent (ESM Table 3) or concurrent study designs (ESM Table 4) (Table 1).
Within 22 studies there was more than one observational, control or intervention group. In 26 studies patient inclusion was restricted to those with risk factors for candidemia. This was most common among studies of non-TA methods of infection prevention with 7 of 17 studies so restricted. Most SDD/SOD studies were published between 1985 and 2000 and most were European in origin (Fig. 2; Table 1).
Scatter plot and linear regression (p = 0.001) of candidemia incidence in observational groups (open black circles) versus year of study publication. Also shown are the control groups from studies of non-TA methods (open blue triangles) and also concurrent control (CC) studies of SDD/SOD (closed red triangles). Note that studies that used candidemia risk factors as a basis for patient selection are not shown and the y-axis is a logit scale
Within six SDD/SOD studies there were four control groups that received an antifungal routinely and five SDD/SOD intervention groups that received an SDD/SOD regimen not containing an antifungal. The incidence of bacteremia was higher among the SDD/SOD control groups from studies with a concurrent design than the observational groups (Table 1).
Candidemia
Among the 54 observational groups, the mean candidemia incidence proportion was 1.5 % (1.2–1.9) overall. This is the candidemia benchmark. The mean candidemia incidence proportion among the observational groups with versus without candidemia risk factors was 3.3 % (2.3–4.7) versus 1.0 % (0.8–1.2), respectively (ESM Fig. 1). The group-level impact of candidemia risk factors and year of publication (Fig. 2) on the candidemia incidence were each significant although European origin was not (Table 2). The mean candidemia incidence proportions among the control groups and also the intervention groups of concurrent design SDD/SOD studies were each higher than the benchmark.
There is an asymmetrical distribution within scatter plots of candidemia incidence for all categories of component group (Fig. 3). This asymmetry is a consequence of a shift to the right among both the control and intervention (Table 1 footnotes p and q) component groups of the concurrent control design studies of SDD/SOD with the candidemia incidence being greater than 1.5 % for 19 of 23 of such non-zero component groups (Fig. 3 and ESM Figs. 5, 6).
Scatter plots of candidemia incidence in component groups (C control; I intervention) from studies of non-TA and SDD/SOD, with non-concurrent (NC) and concurrent control (CC) design, as methods of infection prevention in the ICU (blue symbols). The benchmark candidemia is the summary mean derived from the observation groups (black symbols; central vertical line; as derived in ESM Fig. 1) together with the 95 % prediction intervals (outer vertical lines). Note that the x-axis is a logit scale truncated at 10 % and the groups are not weighted for group size in this display but are in ESM Figs. 2–6. Groups with a zero candidemia count either observed or presumed (imputed) are open symbols (pale blue)
The effects of membership of the various categories of component group together with the effect of group-wide exposures to other factors were examined (Table 2). The effects of membership of either a control or an intervention group of a concurrent control design SDD/SOD study were each significant, positive, and similar in magnitude to that of the negative effect of group-wide exposure to amphotericin and also to that of the significant positive effect of candidemia risk factors used as patient inclusion criteria (Table 2).
The above findings were robust to the following sensitivity tests. Firstly, the base model was repeated using only those intervention studies that had been listed in systematic reviews. Also, as a test for the effect of possible missing SDD/SOD component groups from within the benchmark range, the base model was repeated with all component groups from studies of non-TA methods arbitrarily reclassified as the putative ‘missing’ groups. With these sensitivity tests, the recalculated coefficients were similar and specifically those associated with membership of a component group of a concurrent design SDD/SOD study remained significant and positive (Table 2, footnotes).
Discussion
The findings here from a meta-analysis of candidemia incidence in a broad range of component groups from the literature indicate that there is a contextual (indirect) effect of topical antibiotic within studies of SDD/SOD which is similar in magnitude to the direct effect of conventional risk factors for candidemia and against which protocolized antifungal prophylaxis (PAFP) has an attenuating effect.
The effect of TA as used within SDD/SOD regimens on the incidence of candidemia and invasive candidiasis is of great interest for several reasons. Two recent systematic reviews of randomized concurrent controlled trials have shown that SDD/SOD appears to be protective against yeast colonization [9, 10]. In this regard, the protection obtained with SDD/SOD appears to outperform that obtained by using prophylaxis with azole antifungals in this patient group [10]. Also, SDD/SOD appears to protect against invasive fungal infection [9, 10] and possibly even mortality [10].
On the other hand, SDD/SOD protection against candidemia was not evident in the largest such trial undertaken to date [4]. This trial is notable as being intentionally non-concurrent in design in order to avoid any possible contextual effect on the study outcome.
Moreover, SDD/SOD may have complex ecological effects on the microbiome within the ICU and clarifying the nature and direction of these effects is crucial in defining the role of SDD/SOD going forward [23]. That there are both concurrent and non-concurrent studies of SDD/SOD, that the TA and PAFP constituents within SDD/SOD are each variously constituted, that there are studies of infection prevention methods other than SDD/SOD in this patient group, and that there are observational studies provides a natural experiment with which to test for compound effects of the topical antibiotic and PAFP constituents of SDD/SOD using methods as used in the analysis of cluster randomized trials to test for possible herd effects as found in vaccine trials [13, 15].
There are several challenges and potential study limitations in undertaking the analysis here. Estimating the contextual effects within studies requires a calibration of the observed incidence amongst the component groups of these studies versus an external reference or benchmark range. The benchmark range used here was derived using 54 groups from 36 observational studies. Secondly, candidemia is a rare event. Many studies, especially if small, will have a zero incidence of candidemia but a non-trivial upper 95 % confidence limit which can be approximated by the “rule of three” [24]. For example, the upper 95 % confidence interval for a group of size N = 60 with zero events can be approximated by the “rule of three” as 5 % (=3/N) [24]. Alternately, researchers may have studied a higher-risk population through the use of candidemia risk factors as a basis for patient inclusion [25]. This practice is common among studies of antifungal prophylaxis in these ICU patient populations. The prevalence of these risk factors in a typical ICU may be as low as 10 %, but amongst patients having these risk factors the candidemia incidence is up to fourfold higher [26, 27]. Indeed, in this analysis, the use of candidemia risk factors as criteria for patient inclusion accounts for a 1.6 % (0.8–2.4 %) higher candidema incidence versus the reference group (Table 2). However, adjusting for the group-level presence of various candidemia risk factors in the regression models was not able to explain the higher incidence of candidemia within the studies of SDD/SOD. Moreover, the relative magnitudes of the effect size for the group-level presence of various candidemia risk factors and that of the contextual effect of TA were similar. Thirdly, being a rare outcome, studies of SDD/SOD without a specific report of candidemia incidence should not be dismissed. It should be noted that the majority of the SDD/SOD studies with concurrent control design had less than 50 patients per group and given an expected candidemia incidence of approximately 1 %, a zero event rate would not be unusual. Hence, an important sensitivity analysis undertaken here includes several studies of SDD/SOD which did not declare a candidemia incidence. However, the inclusion of 17 such component groups from among the SDD/SOD studies into the analysis with a presumption that the candidemia incidence in each was zero does not alter the conclusions (Table 2). This presumption of zero incidence would be a “best case scenario” in considering whether or not Candida infection rates might have been inflated by a TA-induced contextual effect.
The literature has been searched and the data extracted by a single author. There remains the possibility that there may be several unpublished or missing studies of SDD/SOD with an incidence of candidemia within the benchmark range that would alter the findings here. As a sensitivity test to this contingency, the component groups from 17 studies of non-TA methods were used as a source of such “missing” studies and were arbitrarily reclassified as component groups of concurrent control SDD/SOD studies. However, the findings in this sensitivity test remain as before.
Of note, the studies included here include all those from the systematic reviews of SDD/SOD studies on fungal colonization and infection which had each demonstrated significant relative risk or odds ratios [9, 10]. Only with calibration is it possible to consider whether these significant ratios represent a relative increase in incidence among the control groups or a relative decrease among the intervention groups. The findings here are broadly consistent with these significant ratios but favor the former inference.
Finally, the group-level analysis undertaken here is unable to take account of patient-specific risk factors. For example, the empiric use of antifungal therapies in each study is an important unknown. The empiric use of antifungals, whether as non-protocolized prophylaxis or as therapy, may counteract any vulnerability to candidemia at the individual level. A further limitation is the uncertain impact of observer blinding. The effect of observer blinding may be confounded by the separate effect of topical placebo in this context [28].
The impacts of known risk factors for candidemia are well established and increase risk by as much as fourfold [26, 27]. While, the possible impact of additional unmeasured and unknown patient-level risk factors for candidemia here remains uncertain, it is unlikely that such unidentified patient-level risk factors would be able to account for the discrepancies noted here. Such a putative patient-level risk factor would need to be at least as strong as the known risk factors for candidemia as identified here and consistently so across all the studies and yet also be profoundly unevenly distributed, predominating in the component groups of the concurrent control SDD/SOD studies versus the other groups.
Candidemia incidence increased with publication year among the groups of the observational studies examined here. Increases in the occurrence of candidemia over a similar time period has also been reported for Dutch [29], US [30], Parisian [31], Brazilian [32], and Italian centers [33]. It remains unclear whether this increase is attributable to changes in recognition, increasing sensitivity of culture methods, emergence of non-albicans Candida, changes in patient populations, or represents a true increase in incidence. In any case, the high incidence within the component groups of the SDD/SOD studies is not explicable by this time trend.
It remains to be determined how the compound effects of SDD/SOD may be mediated. Most invasive infections caused by Candida in the ICU are thought to originate from endogenous flora by translocation [2] and colonization is a key risk factor [34]. The relative importance of origin from cross transmission is uncertain and this requires detailed typing to detect [35–37]. The importance of cross transmission likely varies for different institutions, different time periods, different patient groups, and for non-albicans Candida.
Presumably the TA within SDD/SOD alters the microbiome of the entire ICU, as was postulated in the first study of SDD, whereas topical amphotericin appears to attenuate this process. Of note, complete Candida decolonization using topical amphotericin is difficult to achieve [38, 39]. Regarding the impact of SDD/SOD on the time course of decolonization within Candida-colonized patients receiving MV, decolonization was achieved in only 62 % [38] to 65 % [39] of patients.
The impacts of contextual effects within the ICU on patient outcome are an area of emerging interest. These cannot be estimated within a single-center study [40].
Conclusion
The topical antibiotic and antifungal constituents of SDD/SOD have compound, concurrent, and contextual effects on the incidence of candidemia in the ICU. The results of individual studies require cautious interpretation. The observations in aggregate here add to other paradoxical observations in relation to the incidences of bacteremia and VAP among the SDD/SOD studies which also imply a broad contextual effect of TA as had originally been postulated [3]. The PAFP component of SDD/SOD partially attenuates the contextual effect of TA on candidemia.
Abbreviations
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- SDD:
-
Selective digestive decontamination
- TA:
-
Topical antibiotic
References
Kett DH, Azoulay E, Echeverria PM, Vincent JL (2011) Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 39(4):665–670
Vincent JL, Anaissie E, Bruining H, Demajo W, El-Ebiary M, Haber J, Solomkin J (1998) Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med 24(3):206–216
Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF (1984) The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med 10(4):185–192
de Smet AMGA, Kluytmans JAJW, Cooper BS et al (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev CD000022. doi:10.1002/14651858.CD000022.pub3
Vandenbroucke-Grauls CM, Vandenbroucke JP (1991) Effect of selective decontamination of the digestive tract on respiratory tract infections and mortality in the intensive care unit. Lancet 338:859–862
Hurley JC (1995) Prophylaxis with enteral antibiotics in ventilated patients: selective decontamination or selective cross-infection? Antimicrob Agents Chemother 39(4):941–947
Pileggi C, Bianco A, Flotta D, Nobile CG, Pavia M (2011) Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care 15:R155
Silvestri L, Van Saene HK, Milanese M, Gregori D (2005) Impact of selective decontamination of the digestive tract on fungal carriage and infection: systematic review of randomized controlled trials. Intensive Care Med 31(7):898–910
van Till JO, van Ruler O, Lamme B, Weber RJ, Reitsma JB, Boermeester MA (2007) Single-drug therapy or selective decontamination of the digestive tract as antifungal prophylaxis in critically ill patients: a systematic review. Crit Care 11:R126
Jensen JUS, Hein L, Lundgren B et al (2015) Invasive Candida infections and the harm from antibacterial drugs in critically ill patients: data from a randomized, controlled trial to determine the role of ciprofloxacin, piperacillin-tazobactam, meropenem, and cefuroxime. Crit Care Med 43(3):594–602
Hurley JC (2008) Profound effect of study design factors on ventilator-associated pneumonia incidence of prevention studies: benchmarking the literature experience. J Antimicrob Chemother 61:1154–1161
Hurley JC (2005) Inapparent outbreaks of ventilator-associated pneumonia: an ecologic analysis of prevention and cohort studies. Infect Control Hosp Epidemiol 26:374–390
Hurley JC (2014) Topical antibiotics as a major contextual hazard toward bacteremia within selective digestive decontamination studies: a meta-analysis. BMC Infect Dis 14:714. http://www.biomedcentral.com/content/pdf/s12879-014-0714-x.pdf
Hurley JC (2014) Ventilator associated pneumonia prevention methods using topical antibiotics: herd protection or herd peril? Chest 146(4):890–898
Hurley JC (2015) Candidemia and selective digestive decontamination (SDD) in the ICU: herd protection or herd peril? A meta-analysis. Poster number 314, 19th Congress International Society for Human and Animal Mycology. Melbourne, 3–8 May 2015. http://www.isham2015.com.au/images/isham/ISHAM15%20POSTER%20Author%20List%20.pdf. Accessed 15 June 2015
Greenland S, O’Rourke K (2008) Meta-analysis. In: Rothman KJ, Greenland S, Lash TL (eds) Modern epidemiology, 3rd edn. Lippincott Williams and Wilkins, Philadelphia, pp 652–682
Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altmamn DG, Sterne JAC (2008) Metan: fixed- and random-effects meta-analysis. Stata J 8:3–28
Harbord RM, Higgins JPT (2008) Meta-regression in Stata. Stata J 8:493–519
Mutapi F, Roddam A (2002) P values for pathogens: statistical inference from infectious-disease data. Lancet Infect Dis 2:219–230
Safdar N, Dezfulian C, Collard HR, Saint S (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 33:2184–2193
Chan EY, Ruest A, Meade MO, Cook DJ (2007) Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 334:889–900
Oostdijk EA, de Smet AM, Blok HE et al (2010) Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med 181(5):452–457
Hanley JA, Lippman-Hand A (1983) If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 249:1743–1745
Paphitou NI, Ostrosky-Zeichner L, Rex J (2005) Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycol 43:235–243
Ostrosky-Zeichner L, Sable C, Sobel J, Alexander BD, Donowitz G, Kan V, Rex JH (2007) Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis 26(4):271–276
Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, Wenzel RP, The NEMIS study group (2001) Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis 33(2):177–186
Hurley JC (2013) The perfidious effect of topical placebo: calibration of Staphylococcus aureus ventilator-associated pneumonia incidence within selective digestive decontamination studies versus the broader evidence base. Antimicrob Agents Chemother 57:4524–4531
Voss A, Kluytmans JAJW, Koeleman JGM, Spanjaard L, Vandenbroucke-Grauls CMJE, Verbrugh HA, Meis JFGM (1996) Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur J Clin Microbiol Infect Dis 15(12):909–912
Pittet D, Wenzel RP (1995) Nosocomial bloodstream infections: secular trends in rates, mortality, and contribution to total hospital deaths. Arch Int Med 155(11):1177–1184
Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, French Mycosis Study Group (2014) Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med 40(9):1303–1312
Colombo AL, Guimarães T, Sukienik T, Pasqualotto AC, Andreotti R, Queiroz-Telles F, Nucci M (2014) Prognostic factors and historical trends in the epidemiology of candidemia in critically ill patients: an analysis of five multicenter studies sequentially conducted over a 9-year period. Intensive Care Med 40(10):1489–1498
Bassetti M, Righi E, Costa A, Fasce R, Molinari MP, Rosso R, Viscoli C (2006) Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis 6(1):21
Eggimann P, Pittet D (2014) Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med 40(10):1429–1448
Burnie JP, Odds FC, Lee W, Webster C, Williams JD (1985) Outbreak of systemic Candida albicans in intensive care unit caused by cross infection. BMJ 290(6470):746–748
Bougnoux ME, Kac G, Aegerter P, d’Enfert C, Fagon JY, CandiRea Study Group (2008) Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med 34(2):292–299
Hamal P, Kappe R, Rimek D (2001) Rate of transmission and endogenous origin of Candida albicans and Candida glabrata on adult intensive care units studied by pulsed field gel electrophoresis. J Hosp Infect 49(1):37–42
Ong DS, Klouwenberg PMK, Spitoni C, Bonten MJ, Cremer O (2013) Nebulised amphotericin B to eradicate Candida colonisation from the respiratory tract in critically ill patients receiving selective digestive decontamination: a cohort study. Crit Care 17(5):R233
Van der Geest PJ, Dieters EI, Rijnders B, Groeneveld JA (2014) Safety and efficacy of amphotericin-B deoxycholate inhalation in critically ill patients with respiratory Candida spp. colonization: a retrospective analysis. BMC Infect Dis 14(1):575
Valentin A, Capuzzo M, Guidet B, Moreno RP, Dolanski L, Bauer P, Metnitz PG (2006) Patient safety in intensive care: results from the multinational Sentinel Events Evaluation (SEE) study. Intensive Care Med 32(10):1591–1598
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that he has no competing interests.
Funding
This work was supported by the Australian Government Department of Health and Ageing through the Rural Clinical Training and Support (RCTS) program.
Additional information
Take-home message: There is a contextual (indirect) effect of topical antibiotic on candidemia within studies of SDD/SOD which is similar in magnitude to the direct effect of conventional risk factors for candidemia and against which protocolized antifungal prophylaxis (PAFP) has an attenuating effect. The concurrent design studies of SDD/SOD require cautious interpretation, as the effects are inapparent in any study examined in isolation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hurley, J.C. ICU-acquired candidemia within selective digestive decontamination studies: a meta-analysis. Intensive Care Med 41, 1877–1885 (2015). https://doi.org/10.1007/s00134-015-4004-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4004-x