Abstract
Background
Spontaneous breathing trials (SBT) can be exhausting, but the preventive role of rest has never been studied. This study aimed to evaluate whether reconnection to mechanical ventilation (MV) for 1 h after the effort of a successful SBT could reduce the need for reintubation in critically ill patients.
Methods
Randomized multicenter trial conducted in 17 Spanish medical-surgical intensive care units (Oct 2013–Jan 2015). Patients under MV for longer than 12 h who fulfilled criteria for planned extubation were randomly allocated after a successful SBT to direct extubation (control group) or reconnection to the ventilator for a 1-h rest before extubation (rest group). The primary outcome was reintubation within 48 h. Analysis was by intention to treat.
Results
We recruited 243 patients randomized to the control group and 227 to the rest group. Median time from intubation to SBT did not differ between groups [5.5 (2.7, 9.6) days in the control group vs. 5.7 (2.7, 10.6) in the rest group; p = 0.85]. Reintubation within 48 h after extubation was more common in the control than in the rest group [35 (14%) vs. 12 (5%) patients; OR 0.33; 95% CI 0.16–0.65; p < 0.001]. A multivariable regression model demonstrated that the variables independently associated with reintubation were rest [OR 0.34 (95%CI 0.17–0.68)], APACHE II [OR 1.04 (1.002–1.077)], and days of MV before SBT [OR 1.04 (1.001–1.073)], whereas age, reason for admission, and type and duration of SBT were not.
Conclusion
One-hour rest after a successful SBT reduced the rates of reintubation within 48 h after extubation in critically ill patients.
Trial registration Clinicaltrials.gov identifier NCT01915563.
Similar content being viewed by others
Introduction
Spontaneous breathing trials (SBTs) are used to assess readiness for patients’ liberation from the ventilator [1,2,3,4], but it is difficult to choose the best time to perform the SBT, although an international consensus panel stated several minimum conditions [5]. Moreover, postextubation respiratory failure causes 5–30% of patients to require reintubation, which is associated with higher mortality [6,7,8,9].
Attempts to ascertain the effects of different types of SBT on weaning outcome have yielded inconclusive results. Very demanding tests may better identify suitable patients who will be successfully weaned, but can induce fatigue in very sick patients, thus precluding extubation [10,11,12].
In the vast majority of weaning studies, patients were immediately extubated after successfully passing the SBT, but in other studies, patients were reconnected to the ventilator for variable intervals before extubation. In the 1990s, Ely et al. [13] reported a study in which respiratory therapists performed the SBT, reconnected the patients to the ventilator, and reported to attending physicians about weanability. Their positive results gained wide acceptance, becoming routine clinical practice in some countries, but not in others [14]. In other studies, patients were reconnected to the ventilator for a period after successful SBT to study minute ventilation recovery as a predictor of extubation outcome [15, 16]. These studies inadvertently enabled patients to rest for some time before extubation. However, the effects of rest after the effort of a successful SBT have never been purposely studied. In addition, respiratory muscles can develop high-frequency or low-frequency contractile fatigue (also known as short-lasting and long-lasting fatigue, respectively). Patients can recover from high-frequency fatigue in 10–15 min; however, low-frequency fatigue can persist for more than 24 h, suggesting that the diaphragm could need more than 24 h to recover after an overwhelming effort [17]. Nevertheless, half of the recovery of low-frequency fatigue is accomplished in the first hour. High-frequency fatigue is not a common cause of weaning failure in patients who fulfil standard criteria for weaning, although many patients with weaning failure have diaphragmatic weakness [18].
Given that most ICU patients have some degree of muscle weakness and reduced diaphragm thickness and that the effort induced by SBTs may be excessive for some patients, we hypothesized that a period of rest after a successful SBT and before extubation may be advisable and would reduce postextubation respiratory failure and reintubation. Thus, we aimed to determine whether adding a 1-h rest to standard care would reduce the reintubation rate in critically ill ventilated patients.
This work was presented in the 29th Annual ESICM Congress [19].
Methods
We undertook a parallel, two-arm, prospective randomized controlled clinical trial in 17 Spanish medical-surgical ICUs, from October 2013 to January 2015. The study at the Coordinating Center (Hospital Universitari Mútua Terrassa was approved by the Ethics Committee; approval #1310/signed 22/05/2013). The institutional review board at each center approved the protocol, and patients or their authorized representatives provided written informed consent.
Study population
Patients receiving invasive mechanical ventilation (MV) for at least 12 h were screened daily and followed prospectively (while undergoing serial SBTs) until they successfully completed an SBT. Criteria for assessing weanability by SBT were based on the literature (see supplementary material) [4]. Exclusion criteria were age less than 18 years, tracheostomy, overwhelming respiratory secretions, inability to follow commands, do-not-resuscitate or do-not-reintubate orders, out-of-protocol extubation, participation in other trials, or formal indication for noninvasive ventilation (NIV) after extubation, mainly due to hypercapnia during SBT.
Randomization and masking
Randomization was centralized and stratified by center and by risk of extubation failure before SBT. It was achieved via computerized random-number tables in blocks of four for each hospital and it was unknown by the investigators involved in recruiting patients. Allocation was concealed through numbered opaque envelopes.
To reduce bias, investigators who collected endpoints were excluded from clinical decisions. Because it was impossible to mask patients and staff to treatment, the database was monitored by third parties with no direct involvement in the study and no interest in the outcome, and the data were analyzed exactly according to the statistical analysis plan decided on before the study started.
Procedures
Patients receiving invasive MV for at least 12 h were screened daily. When patients fulfilled criteria for assessing weanability, informed consent was obtained and patients were randomized before the SBT. To avoid unbalanced distribution, patients were classified before the SBT according to predicted risk of extubation failure as high or low risk [20,21,22] (see supplementary material).
The technique for the SBT [T-tube, low-level pressure support, or continuous positive airway pressure (CPAP)] and duration (30, 60, or 120 min) remained at the discretion of the attending physician and local protocols. Successful SBT was defined by international guidelines [4] (see supplementary material).
As per our standard of care, patients who passed the SBT were directly extubated if assigned to the control group or were reconnected to the ventilator with the previous ventilatory parameters for 1-h rest and then directly extubated if assigned to the rest group.
Each participating hospital used its own weaning and physiotherapy protocols, so practices such as pre-oxygenation before extubation, suctioning before and/or during cuff deflation, using bronchodilators, and oxygen supply after extubation varied from one center to another.
Postextubation respiratory failure was defined according to the literature (see supplementary material) [22]. The protocol at each participating hospital determined the treatment of postextubation respiratory failure. Reintubation and use of NIV as ventilatory support after extubation remained at the discretion of the attending physicians.
Patients were followed up until hospital discharge or death.
The primary outcome was reintubation within 48 h. Secondary endpoints were postextubation respiratory failure, ICU and hospital length of stay, and ICU and hospital mortality.
Statistical analysis
We expected a 15% reintubation rate in the control group, and we aimed to detect a 5% decrease (33% relative reduction) in the rest group. This objective required a sample of 686 patients in each group (i.e., 1372 in total) for an alpha error of 5% and a power level (1 − β) of 80%.
All analyses were conducted adhering to the intention-to-treat principle. Categorical variables were compared with the Cochran–Mantel–Haenszel χ2 test or Fisher’s exact test. Normally distributed continuous variables were expressed as means with standard deviations and compared with Student’s t test for independent samples. Non-normally distributed continuous variables were expressed as medians with 25% and 75% percentiles and compared with the Mann–Whitney U test.
To identify independent factors related to reintubation and control for confounding variables, we constructed a conditional backwards stepwise multivariable logistic regression model including the pre-specified variables age, APACHE II score, reason for admission, type and duration of SBT, length of MV before SBT, and reconnection to the ventilator for rest, and those independent variables which are not distributed evenly between the two groups of study (p < 0.05). The discrimination of the multivariate model was assessed using the area under the receiver operating characteristic curve (AUROC) and the goodness-of-fit by Hosmer–Lemeshow test. We assessed the sensitivity of our findings by repeating the primary analysis under varying assumptions about the study population in a sensitivity analysis for reintubation (see supplementary material).
Primary outcome was extubation failure. Secondary outcomes were reintubation, ICU and hospital length of stay, and ICU and hospital mortality. Significance was set at 0.05 and statistical analyses were conducted by the medical statistical department of the Hospital Universitari Mutua de Terrassa (SPSS, version 17.0; SPSS Inc, Chicago, IL, USA).
This trial was registered at clinicaltrials.gov (NCT01915563).
Results
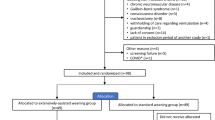
Figure 1 shows the flowchart of the study. We admitted 4317 patients needing MV, and 2765 of these were ventilated for more than 12 h (thereby excluding patients with immediate, uncomplicated postoperative extubation). Finally, 608 patients were eligible for the study and 470 of these were randomized: 243 patients to the control group and 227 to the rest group. No patients declined to participate after providing consent, and none were lost to follow-up. Only after concluding the study did we realize that we had miscalculated the sample size as 470 patients instead of the 1372 stated in the “Methods” section.
The characteristics and severity of illness of patients in the two groups were similar (Table 1). The median length of MV before SBT was not different between groups [5.5 (2.7, 9.6) days in the control group vs. 5.7 (2.7, 10.6) days in the rest group (p = 0.85)].
The most common SBT technique was T-tube in both groups [211 (87%) patients in the control group and 213 (94%) in the rest group]; only 43 (9.1%) patients received pressure support or CPAP. The duration of the SBTs was evenly distributed among 30-, 60-, and 120-min time frames [162 (34.5%), 184 (39.1%), and 124 (26.4%), respectively]. SBTs in the 120-min time frame were more frequent in the control than in the rest group [77 (31.7%) vs. 47 (20.7%); p = 0.009].
In the rest group, 9 (3.7%) patients [3 (1.5%) high-risk patients and 6 (11%) low-risk patients] did not tolerate reconnection to the ventilator; intolerance was described as a general sense of agitation. All these patients were directly extubated, and none failed extubation; they remained in the rest group for the intention-to-treat analysis.
Postextubation respiratory failure within 48 h occurred in 82 (17%) patients and was more common in the control group than in the rest group [58 (24%) vs. 24 (10%) patients; OR 0.35 (0.21–0.61); p < 0.001] (Table 2).
Among these 82 patients, 47 (57%) patients underwent rescue NIV [34 (59%) in the control group and 13 (54%) in the rest group; p = 0.71] for a median of 12 h (25th–75th centiles, 6–30 h), whereas 31 (40%) patients were directly reintubated [22 (38%) patients in the control group vs. 9 (37%) in the rest group; p = 0.97]. Four patients (5%) were treated solely with oxygen and physiotherapy. Reintubation was eventually necessary in 16 (34%) patients who received NIV (13 (38%) patients in the control group and 3 (23%) in the rest group; p = 0.33) (Table 2).
Reintubation within 48 h after extubation, either direct reintubation or intubation after rescue NIV, was more common in the control group than in the rest group [35 (14%) vs. 12 (5%); OR 0.33; 95% CI 0.16–0.65; p < 0.001] (Fig. 2). In both groups, the main reason for reintubation was patients’ inability to manage secretions that eventually induced acute respiratory failure (Table 2).
Reintubation within 48 h after extubation was more frequent in high-risk patients [43/392 (11%) vs. 4/78 (5%); p = 0.001], both in the control group [32/202 high-risk patients (16%) vs. 3/41 low-risk patients (7%); p = 0.001] and in the rest group [11/190 high-risk patients (6%) vs. 1/37 low-risk patients (3%); p = 0.001].
The median length of ICU stay was not different between groups [10 (5–19) days in the control group vs. 11 (6–18) days in the rest group; p = 0.30]. The median length of hospital stay was not different between groups [23 (14–38) days in the control group vs. 26 (17–43) days in the rest group; p = 0.93].
Eight patients (2%) died in the ICU during the 48-h postextubation period after developing postextubation respiratory failure; there were no differences in mortality between groups. Only high-risk patients died: six patients were directly reintubated after postextubation respiratory failure and the other two were reintubated within 48 h after extubation, but after several hours of NIV. Another 14 patients died in the ICU beyond 48 h for unrelated reasons, such as postsurgical complications or cardiac arrest.
A multivariable regression model demonstrated that the variables independently associated with reintubation were rest [OR 0.34 (95%CI 0.17–0.68); p = 0.002], APACHE II on admission [OR 1.04 (1.002–1.077); p = 0.04], and days of MV before SBT [OR 1.04 (1.001–1.073); p = 0.04]; age, reason for admission, and type and duration of SBT were discarded (see supplementary material).
Discussion
Our main finding is that reconnection to the ventilator to rest for 1 h after a successful SBT reduces reintubation at 48 h in critically ill patients. Postextubation respiratory failure within 48 h was more common in the control group than in the rest group, but no differences were observed in the duration of ICU stay or hospital stay.
We studied a mixed population that included surgical and medical patients at high and low risk of extubation failure, and there were no differences between study groups.
The median MV time before SBT was 5 days in both groups. MV can have deleterious effects on the diaphragm, and muscle unloading and inactivity lead to a condition referred to as “ventilator-induced diaphragmatic dysfunction” [23]. In fact, autopsy studies of brain-dead donors have shown diaphragm myofiber atrophy, a phenomenon attributed to complete diaphragm inactivity that is evident within the first 3 days of MV [24]. A recent study measuring changes in diaphragmatic thickness and contractility by ultrasound found a more than 10% decrease in diaphragmatic thickness in nearly half of the patients during the first week of MV which was associated with lower contractile activity [25]. Diaphragmatic contractile activity varied widely for each patient and from patient to patient over the first week of MV. Therefore it is likely that our patients had varying levels of muscle dysfunction, although we have no direct measurements to support this theory.
SBTs could be very demanding for some critically ill patients. SBTs can be performed without any ventilatory support (T-tube) or with minimal support (5–7 cmH2O pressure support with or without positive end-expiratory pressure or about 5 cmH2O CPAP) [6, 26]. In our clinical scenario, physicians mostly opted for T-tube SBTs for 30–60 min, commonly accepted as equally effective at achieving successful extubation [9].
Another important factor for respiratory muscle function is fatigue. Fatigue involves two components: high-frequency fatigue, which can be resolved in 10–15 min, and low-frequency fatigue, which can persist for more than 24 h. Unfortunately, the lack of physiological data in our study does not allow us to know why we have obtained such striking results. We can only speculate about the mechanisms for improvement with rest on the basis of previous studies. In their study in healthy volunteers, Laghi et al. [17] found that diaphragm fatigue was mainly due to low-frequency fatigue and needed more than 24 h to fully recover. In the same study, the greatest recovery of the diaphragm occurred in the first hour of resting. However, they could not show that patients under MV developed low-frequency fatigue; they speculated that this was due to greater recruitment of the rib cage and expiratory muscles and to patients being reconnected to the ventilator for clinical signs of distress before fatigue developed [18]. Probably, the work of breathing load imposed by SBT is not high enough to fatigue the diaphragm in patients who pass it, but some of these patients could be weak enough to experience failure hours after extubation. Thus, we hypothesize that resting for 1 h could allow those patients at risk of fatigue to recover enough to avoid respiratory distress in the postextubation period.
Tolerance to reconnection to the ventilator was excellent, and the vast majority of patients showed no signs of severe intolerance, described as a general sense of agitation, even under assist-control mode. Only a few low-risk patients did not tolerate reconnection, probably because younger patients without heart or lung disease who fully recovered after a short MV period are unlikely to find relief in returning to the ventilator and more likely refuse to be reconnected.
NIV as a rescue treatment for postextubation respiratory failure has raised safety concerns because it could mask deterioration, thus delaying reintubation. Whereas some studies strongly argued against NIV in this scenario [27], others reported beneficial effects, mainly in hypercapnic patients [28]. In our study, “as reflected in real life”, up to 60% of patients received rescue NIV after failed extubation for a median of 12 h. Finally, 34% of these patients needed reintubation, slightly better than in Esteban et al.’s [27] study, but mortality was not worse in our study. Our data do not shed light on the value of NIV in postextubation respiratory failure. Moreover, other therapies such as high-flow nasal cannula were not used when this study was done as a result of the lack of evidence.
The reintubation rate in the control group was 14%, which is very similar to rates reported in other studies [29, 30]. Therefore, the decrease in the reintubation rate to 5% after reconnection to MV for rest for 1 h before extubation is highly relevant for clinical practice. It is especially important since it is a simple measure applicable in a wide variety of settings, as our study took place in different hospitals, with different weaning protocols, different nurse/patient ratios, and different levels of physiotherapy support. Furthermore, in many centers, especially in North America, nurses perform the SBT and then physicians decide whether to order extubation, so they inadvertently allow rest for variable periods before extubation. Importantly, our results suggest that caution is advisable when critically reviewing multicenter trials on weaning outcome, where the rate of patient resting before extubation should be a critical parameter.
In both groups in our study, tachypnea and clinical signs of muscle exhaustion were the most common criteria for diagnosing postextubation respiratory failure, and patients’ inability to manage secretions was the most common reason for reintubation. This is in accordance with previous studies about predictors of extubation failure, where cough strength and amount of endotracheal secretions were important predictors of extubation outcomes [19, 31]. In the daily screening, we excluded patients with copious secretions or inability to cough, but we have no data about mucus viscosity or specific respiratory physiotherapy measures after extubation.
Despite the reduction in reintubation in the rest group, mortality was not different between the groups, mainly because death was related to conditions appearing very late after extubation, such as postsurgical complications, and the low number of deaths makes it difficult to detect statistical differences.
Study limitations
After concluding the study, we realized that our original sample size calculation was mistaken as a result of accepting a default power of only 35%, instead of the commonly accepted 80% (obtained via https://www.stat.ubc.ca/~rollin/stats/ssize/b2.html). Then, instead of 225 patients per group, the study should have recruited 686 patients per group. Nevertheless, because the size of the effect was much larger than hypothesized we found a statistically significant reduction in both postextubation respiratory failure and reintubation.
Although different types and durations of SBT between groups might be confounding factors, the multivariable regression analysis dropped them as variables associated with reintubation.
On the other hand, we did not protocolize weaning, extubation, or postextubation care; we did not routinely analyze arterial blood gases and maximal inspiratory pressure; and we have no data about work of breathing during the SBT. Nevertheless, our pragmatic approach is a reflection of daily clinical practice in different centers with different approaches to postextubation respiratory distress, thus improving the external validity of our study. Moreover, like many studies on MV, our study was not blinded.
Whether the beneficial effect of rest may be an alternative to high-flow nasal cannula after extubation or may have additive effects remains speculative and open for future research.
While overt fatigue is commonly recognized in failed SBTs, subclinical fatigue may go undetected during successful SBTs. We did not directly measure the extent of the recovery achieved by patients during the resting period after reconnection to the ventilator, relying on the physicians’ perception of patients’ comfort. Moreover, a small percentage of patients may have developed asynchronies that reduced muscular rest. Finally, reconnection to the ventilator may also help patients recover from atelectasis and derecruitment developed during the SBT.
Conclusions
Allowing patients to rest for 1 h after a successful SBT reduces reintubation and postextubation respiratory failure in critically ill patients. Since this approach requires no special monitoring or respiratory equipment, no additional expenditure, and no laboratory studies, it can be readily implemented in any ICU in both university and community hospitals. As a result of the paucity of physiological data supporting this approach, further studies that would look into the optimal duration of rest and the type of patients who should benefit most are needed.
References
Tobin MJ, Perez W, Guenther SM et al (1986) The pattern of breathing during successful and unsuccessful trials of weaning from mechanical ventilation. Am Rev Respir Dis 134:1111–1118
DeHaven CB Jr, Hurst JM, Branson RD (1986) Evaluation of two different extubation criteria: attributes contributing to success. Crit Care Med 14:92–94
Millbern SM, Downs JB, Jumper LC, Modell JH (1978) Evaluation of criteria for discontinuing mechanical ventilatory support. Arch Surg 113:1441–1443
Boles JM, Bion J, Connors A et al (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
MacIntyre NR, Cook DJ, Ely EW Jr et al (2001) Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 120(6 Suppl):375S–395S
MacIntyre N (2007) Discontinuing mechanical ventilatory support. Chest 132:1049–1056
Frutos-Vivar F, Esteban A, Apezteguia C et al (2011) Outcome of reintubated patients after scheduled extubation. J Crit Care 26:502–509
Epstein S, Ciubotaru RL, Wong JB (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112:186–192
Esteban A, Alía I, Tobin MJ et al (1999) Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med 159:512–518
Bolder PM, Healy TE, Bolder AR, Beatty PC, Kay B (1986) The extra work of breathing through adult endotracheal tubes. Anesth Analg 65:853–859
Schweickert W, Pohlman M, Pohlman A et al (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 373:1874–1882
Uchiyama A, Yoshida T, Yamanaka H, Fujino Y (2013) Estimation of tracheal pressure and imposed expiratory work of breathing by the endotracheal tube, heat and moisture exchanger and ventilator during mechanical ventilation. Respir Care 58:1157–1169
Ely EW, Bennett P, Bowton D, Murphy S, Florance A, Haponik E (1999) Large implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med 159:439–446
Blackwood B, Burns KEA, Cardwell CR, O’Halloran P (2014) Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 11:CD006904
Martínez A, Seymour C, Nam M (2003) Minute ventilation recovery time. A predictor of extubation outcome. Chest 123:1214–1221
Hernandez G, Fernandez R, Luzon E, Cuena R, Montejo JC (2007) The early phase of the minute ventilation recovery curve predicts extubation failure better than the minute ventilation recovery time. Chest 131:1315–1322
Laghi F, D’Alfonso N, Tobin MJ (1995) Pattern of recovery from diaphragmatic fatigue over 24 h. J Appl Physiol 79:539–546
Laghi F, Cattapan SE, Jubran A et al (2003) Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167:120–127
Fernandez MM, Fernandez R, Magret M et al (2016) Reconnection to mechanical ventilation for 1 h after a successful spontaneous breathing trial reduces extubation failure and reintubation in critically ill patients: a multicenter randomised controlled trial. Intensive Care Med Exp 4(Suppl 1: part two):470
Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L (2011) Outcomes of extubation failures in medical intensive care unit patients. Crit Care Med 39:2612–2618
Thille AW, Boissier F, Ben Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C (2015) Risk factors for and prediction by caregivers of extubation failure in ICU patients: a prospective study. Crit Care Med 43:613–620
Hernández G, Vaquero C, Colinas L et al (2016) Effect of postextubation high-flow nasal cannula vs. noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 316:1565–1574
Vassilakopoulos T, Petrof BJ (2004) Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care 169:336–341
Levine S, Nguyen T, Taylor N et al (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Goligher EC, Fan E, Herridge MS et al (2015) Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med 192:1080–1088
Esteban A, Alia I, Gordo F et al (1997) Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. Am J Respir Crit Care Med 156:459–465
Esteban A, Frutos-Vivar F, Ferguson ND et al (2004) Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 350:2452–2460
Ferrer M, Esquinas A, Arancibia F et al (2003) Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 168:70–76
Thille AW, Richard JCM, Brochard L (2013) The decision to extubate in the intensive care unit. Am J Respir Crit Care Med 187:1294–1302
Esteban A, Frutos F, Tobin MJ et al (1995) A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 332:345–350
Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA (2001) Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest 120:1262–1270
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: participating study investigators
Appendix: participating study investigators
Laura Claverias MD, Department of Intensive Care, H. Universitari Joan XXIII, Tarragona, Spain; Juan Pablo Castañeda MD, Department of Intensive Care, H. Verge de la Cinta de Tortosa, Tarragona, Spain; Samantha Huidobro MD, Department of Intensive Care, Hospital Universitario de Canarias, Tenerife, Spain; Diego de Mendoza MD, Department of Intensive Care, Consorci Sanitari Integral Moisés Broggi, Barcelona, Spain; Elena González-Higueras MD, Department of Intensive Care, Hospital Virgen de la Luz-SESCAM, Cuenca, Spain; Lorena Palacios MD, Juan Carlos Sanchís MD, Department of Intensive Care, Hospital Clínico de Valencia, Valencia, Spain; Cristina Pedrós MD, Department of Intensive Care, Hospital General de Vic, Barcelona, Spain; Lorena del Río MD Department of Intensive Care, Complexo Hospitalario Universitario de Ourense, Ourense, Spain; Alberto Belenguer MD Department of Intensive Care, Hospital General Universitario de Castellón, Castellón, Spain.
Rights and permissions
About this article
Cite this article
Fernandez, M.M., González-Castro, A., Magret, M. et al. Reconnection to mechanical ventilation for 1 h after a successful spontaneous breathing trial reduces reintubation in critically ill patients: a multicenter randomized controlled trial. Intensive Care Med 43, 1660–1667 (2017). https://doi.org/10.1007/s00134-017-4911-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4911-0