Abstract
Introduction and hypothesis
The objective of this study was to characterize the bacterial biofilm on vaginal ring pessaries used to treat pelvic organ prolapse and investigate the relationship between biofilm phenotype and patient symptoms and clinical signs that are suggestive of inflammation.
Methods
This was a cross-sectional observational study of 40 women wearing a ring-shaped pessary continuously for at least 12 weeks. Participants underwent a clinical examination, and the pessary was removed. Clinical signs were recorded. A swab from the pessary surface and a high vaginal swab were collected from each woman. Participants completed a questionnaire on symptoms. Pessary biofilm presence and phenotype were determined by scanning electron microscopy (SEM). Vaginal and pessary bacterial composition was determined by 16S rRNA gene sequencing. The relationship between biofilm phenotype and symptoms and clinical signs was assessed using logistic regression.
Results
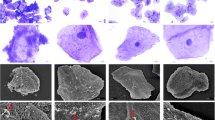
SEM confirmed biofilm formation on all 40 pessaries. Microbiota data were available for 25 pessary swabs. The pessary biofilm microbiota was composed of bacteria typically found in the vagina and was categorized into Lactobacillus-dominated (n = 10/25 pessaries, 40%) communities and Lactobacillus-deficient communities with high relative abundance of anaerobic/facultative anaerobes (n = 15/25 pessaries, 60%). While increasing age was associated with presence of a Lactobacillus-deficient pessary biofilm (odds ratio = 3.60, 95% CI [1.16–11.22], p = 0.04), no associations between biofilm microbiota composition and symptoms or clinical signs were observed.
Conclusions
Lactobacillus-deficient biofilms commonly form on pessaries following long-term use. However, the contribution of biofilm phenotype to symptoms and clinical signs remains to be determined.


Similar content being viewed by others
References
Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186(6):1160–6. https://doi.org/10.1067/mob.2002.123819.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. https://doi.org/10.1016/S0029-7844(97)00058-6.
Obstetricians ACo, Bulletins-Gynecology GCoP, Society AU. Pelvic organ prolapse: ACOG practice bulletin, number 214. Obstet Gynecol. 2019;134(5):e126–42. https://doi.org/10.1097/AOG.0000000000003519.
Sarma S, Ying T, Moore KH. Long-term vaginal ring pessary use: discontinuation rates and adverse events. BJOG. 2009;116(13):1715–21. https://doi.org/10.1111/j.1471-0528.2009.02380.x.
Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881–90. https://doi.org/10.3201/eid0809.020063.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–22. https://doi.org/10.1126/science.284.5418.1318.
Hardy L, Cerca N, Jespers V, Vaneechoutte M, Crucitti T. Bacterial biofilms in the vagina. Res Microbiol. 2017;168(9-10):865–74. https://doi.org/10.1016/j.resmic.2017.02.001.
Trautner BW, Darouiche RO. Catheter-associated infections: pathogenesis affects prevention. Arch Intern Med. 2004;164(8):842–50. https://doi.org/10.1001/archinte.164.8.842.
Swidsinski A, Mendling W, Loening-Baucke V, Swidsinski S, Dorffel Y, Scholze J, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97 e91-96. https://doi.org/10.1016/j.ajog.2007.06.039.
Kalia N, Singh J, Kaur M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob. 2020;19(1):5. https://doi.org/10.1186/s12941-020-0347-4.
Gunawardana M, Moss JA, Smith TJ, Kennedy S, Kopin E, Nguyen C, et al. Microbial biofilms on the surface of intravaginal rings worn in non-human primates. J Med Microbiol. 2011;60(Pt 6):828–37. https://doi.org/10.1099/jmm.0.028225-0.
Fregosi NJ, Hobson DTG, Kinman CL, Gaskins JT, Stewart JR, Meriwether KV. Changes in the vaginal microenvironment as related to frequency of pessary removal. Female Pelvic Med Reconstr Surg. 2018;24(2):166–71. https://doi.org/10.1097/SPV.0000000000000520.
Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005;193(1):103–13. https://doi.org/10.1016/j.ajog.2004.12.025.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36. https://doi.org/10.1007/s11136-011-9903-x.
Plummer EL, Vodstrcil LA, Danielewski JA, Murray GL, Fairley CK, Garland SM, et al. Combined oral and topical antimicrobial therapy for male partners of women with bacterial vaginosis: acceptability, tolerability and impact on the genital microbiota of couples - a pilot study. PLoS One. 2018;13(1):e0190199. https://doi.org/10.1371/journal.pone.0190199.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17(1):10–2.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. https://doi.org/10.1038/nmeth.f.303.
Schmieder R, Lim YW, Rohwer F, Edwards R. TagCleaner: identification and removal of tag sequences from genomic and metagenomic datasets. BMC Bioinformatics. 2010;11:341. https://doi.org/10.1186/1471-2105-11-341.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. https://doi.org/10.1038/nmeth.3869.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–6. https://doi.org/10.1093/nar/gks1219.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2016) vegan: Community Ecology Package. R package version 2.4-1 edn.,
Rohart F, Gautier B, Singh A, Le Cao KA. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11):e1005752. https://doi.org/10.1371/journal.pcbi.1005752.
Hardy L, Jespers V, De Baetselier I, Buyze J, Mwambarangwe L, Musengamana V, et al. Association of vaginal dysbiosis and biofilm with contraceptive vaginal ring biomass in African women. PLoS One. 2017;12(6):e0178324. https://doi.org/10.1371/journal.pone.0178324.
Miller L, MacFarlane SA, Materi HL. A scanning electron microscopic study of the contraceptive vaginal ring. Contraception. 2005;71(1):65–7. https://doi.org/10.1016/j.contraception.2004.07.015.
Crucitti T, Hardy L, van de Wijgert J, Agaba S, Buyze J, Kestelyn E, et al. Contraceptive rings promote vaginal lactobacilli in a high bacterial vaginosis prevalence population: a randomised, open-label longitudinal study in Rwandan women. PLoS One. 2018;13(7):e0201003. https://doi.org/10.1371/journal.pone.0201003.
Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450–8. https://doi.org/10.1097/GME.0b013e3182a4690b.
Hillier SL, Lau RJ. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin Infect Dis. 1997;25(Suppl 2):S123–6. https://doi.org/10.1086/516221.
Heinemann C, Reid G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can J Microbiol. 2005;51(9):777–81. https://doi.org/10.1139/w05-070.
Gliniewicz K, Schneider GM, Ridenhour BJ, Williams CJ, Song Y, Farage MA, et al. Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front Microbiol. 2019;10:193. https://doi.org/10.3389/fmicb.2019.00193.
Alnaif B, Drutz HP. Bacterial vaginosis increases in pessary users. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(4):219–22; discussion 222-213. https://doi.org/10.1007/pl00004026.
Acknowledgments
The authors would like to acknowledge Saima Wani and Georgia Privato for contributions towards specimen collection and Marin Poljak for contributions towards laboratory work.
Funding
This work was supported by a grant from the Urogynaecology Special Purpose Fund, The Royal Women’s Hospital, Melbourne, Australia.
Author information
Authors and Affiliations
Contributions
F. G. Gould: Protocol/Project development, Data collection and management, Data analysis, Manuscript writing/editing.
M. P. Carey: Protocol/Project development, Manuscript writing/editing,
E. L. Plummer: Data analysis, Manuscript writing/editing.
G. L. Murray: Laboratory/experimental analysis, Manuscript writing/editing.
J. A. Danielewski: Laboratory/experimental analysis, Manuscript editing.
S. N. Tabrizi: Protocol/Project development, Manuscript editing.
S. M. Garland: Protocol/Project development, Manuscript editing.
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 24 kb)
ESM 2
(DOCX 24 kb)
Figure S1
The microbiota composition of all pessary (blue) and vaginal specimens included in this study. The heatmap displays the relative abundance of the 15 most abundant taxa detected in all specimens. Hierarchical clustering of Jaccard distances with Ward linkage was used to generate the heatmap and demonstrates similarity of microbiota composition between specimens. The metadata above the heatmap indicate the participant ID and sample type (pessary and vaginal). Pessary and vaginal samples collected from the same woman frequently appear next to each other in the heatmap, indicating similarity in microbiota composition at the two sites. (PDF 28 kb)
Figure S2
Principal component analysis of pessary (blue) and vaginal (orange) samples included in this study. Pessary and vaginal samples collected from the same woman frequently cluster together (or overlap) in the plot, indicating similarity in microbiota composition at the two sites. Pessary samples are labeled with P followed by the study ID and vaginal samples are labeled with V followed by the study ID (i.e., P1 and V1 represent the pessary and vaginal samples collected from participant 1, respectively). (PDF 5 kb)
ESM 5
(DOCX 19 kb)
ESM 6
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Gould, F.G., Carey, M.P., Plummer, E.L. et al. Bacterial biofilm formation on vaginal ring pessaries used for pelvic organ prolapse. Int Urogynecol J 33, 287–295 (2022). https://doi.org/10.1007/s00192-021-04717-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04717-x