Abstract
Summary
We tested whether GPRC6A, the putative receptor of undercarboxylated osteocalcin (ucOC), is present in mouse muscle and whether ucOC increases insulin sensitivity following ex vivo muscle contraction. GPPRC6A is expressed in mouse muscle and in the mouse myotubes from a cell line. ucOC potentiated the effect of ex vivo contraction on insulin sensitivity.
Introduction
Acute exercise increases skeletal muscle insulin sensitivity. In humans, exercise increases circulating ucOC, a hormone that increases insulin sensitivity in rodents. We tested whether GPRC6A, the putative receptor of ucOC, is present in mouse muscle and whether recombinant ucOC increases insulin sensitivity in both C2C12 myotubes and whole mouse muscle following ex vivo muscle contraction.
Methods
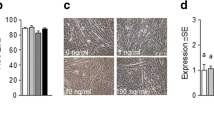
Glucose uptake was examined in C2C12 myotubes that express GPRC6A following treatment with insulin alone or with insulin and increasing ucOC concentrations (0.3, 3, 10 and 30 ng/ml). In addition, glucose uptake, phosphorylated (p-)AKT and p-AS160 were examined ex vivo in extensor digitorum longus (EDL) dissected from C57BL/6J wild-type mice, at rest, following insulin alone, after muscle contraction followed by insulin and after muscle contraction followed by recombinant ucOC then insulin exposure.
Results
We observed protein expression of the likely receptor for ucOC, GPRC6A, in whole muscle sections and differentiated mouse myotubes. We observed reduced GPRC6A expression following siRNA transfection. ucOC significantly increased insulin-stimulated glucose uptake dose-dependently up to 10 ng/ml, in differentiated mouse C2C12 myotubes. Insulin increased EDL glucose uptake (∼30 %, p < 0.05) and p-AKT and p-AKT/AKT compared with rest (all p < 0.05). Contraction prior to insulin increased muscle glucose uptake (∼25 %, p < 0.05), p-AKT, p-AKT/AKT, p-AS160 and p-AS160/AS160 compared with contraction alone (all p < 0.05). ucOC after contraction increased insulin-stimulated muscle glucose uptake (∼12 % p < 0.05) and p-AS160 (<0.05) more than contraction plus insulin alone but without effect on p-AKT. In the absence of insulin and/or of contraction, ucOC had no significant effect on muscle glucose uptake.
Conclusions
GPRC6A, the likely receptor of osteocalcin (OC), is expressed in mouse muscle. ucOC treatment augments insulin-stimulated skeletal muscle glucose uptake in C2C12 myotubes and following ex vivo muscle contraction. ucOC may partly account for the insulin sensitizing effect of exercise.







Similar content being viewed by others
References
Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ (2010) Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42:2282–2303
Holloszy JO (2005) Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 99:338–343
Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD (2009) Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297:E242–251
Lee NK, Sowa H, Hinoi E et al (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Ducy P (2011) The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54:1291–1297
Karsenty G, Ferron M (2012) The contribution of bone to whole-organism physiology. Nature 481:314–320
Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B (2009) Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 94:827–832
Rached MT, Kode A, Silva BC et al (2010) FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest 120:357–368
Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308
Oury F, Sumara G, Sumara O et al (2011) Endocrine regulation of male fertility by the skeleton. Cell 144:796–809
Levinger I, Zebaze R, Jerums G, Hare DL, Selig S, Seeman E (2011) The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos Int 22:1621–1626
Levinger I, Jerums G, Stepto NK et al (2014) The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. J Bone Miner Res 29:2571–2576
Wan M, Birnbaum Morris J (2011) Of mice and men: not ExAKTly the same? Cell Metab 14:722–723
Cartee GD, Wojtaszewski JF (2007) Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32:557–566
Krook A, Wallberg-Henriksson H, Zierath JR (2004) Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc 36:1212–1217
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A 105:5266–5270
Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD (2010) In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab 298:E999–1010
Merry TL, Steinberg GR, Lynch GS, McConell GK (2010) Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab 298:E577–585
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G (2012) Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 50:568–575
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci 105:5266–5270
Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL, Lin A, Lam KS (2011) Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes 60:486–495
Mollica JP, Oakhill JS, Lamb GD, Murphy RM (2009) Are genuine changes in protein expression being overlooked? Reassessing Western blotting. Anal Biochem 386:270–275
Richter EA, Derave W, Wojtaszewski JF (2001) Glucose, exercise and insulin: emerging concepts. J Physiol 535:313–322
Brennan-Speranza TC, Henneicke H, Gasparini SJ et al (2012) Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest 122:4172–4189
Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ (2006) Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55:2067–2076
Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ (2006) AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281:31478–31485
Treebak JT, Pehmoller C, Kristensen JM, Kjobsted R, Birk JB, Schjerling P, Richter EA, Goodyear LJ, Wojtaszewski JF (2014) Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J Physiol 592:351–375
Oury F, Ferron M, Huizhen W et al (2013) Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest 123:2421–2433
Pi M, Quarles LD (2012) Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology 153:2062–2069
Wellendorph P, Brauner-Osborne H (2004) Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335:37–46
Pi M, Chen L, Huang MZ et al (2008) GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 3:e3858
Hill HS, Grams J, Walton RG, Liu J, Moellering DR, Garvey WT (2014) Carboxylated and uncarboxylated forms of osteocalcin directly modulate the glucose transport system and inflammation in adipocytes. Horm Metab Res 46:341–347
Wojtaszewski JF, Hansen BF, Gade KB, Markuns JF, Goodyear LJ, Richter EA (2000) Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49:325–331
Acknowledgments
A/Prof Itamar Levinger is a Heart Foundation Future Leader Fellow (ID: 100040), and this manuscript represents a collaboration between The University of Melbourne and Victoria University as part of the Collaborative Research Network (CRN) programme. This study was funded by Diabetes Australia Research Trust (DART). We thank Professor Gerard Karsenty and Professor Mathieu Ferron for the gift of recombinant ucOC. We also thank Dr Raul Bescós for assisting with the experiment.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Levinger and X. Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Levinger, I., Lin, X., Zhang, X. et al. The effects of muscle contraction and recombinant osteocalcin on insulin sensitivity ex vivo. Osteoporos Int 27, 653–663 (2016). https://doi.org/10.1007/s00198-015-3273-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3273-0