Abstract
Summary
The role of exercise in preventing osteoporotic fractures is vague, and further recommendations for optimized exercise protocols are very rare. In the present work, we provided positive evidence for exercise effects on the number of osteoporotic fractures in adults, albeit without observing any significant relevance of intensity progression or study duration.
Introduction
Osteoporotic fractures are a major challenge confronting our aging society. Exercise might be an efficient agent for reducing osteoporotic fractures in older adults, but the most promising exercise protocol for that purpose has yet to be identified. The present meta-analysis thus aimed to identify important predictors of the exercise effect on osteoporotic fractures in adults.
Methods
We conducted a systematic search of six literature databases according to the PRISMA guideline that included controlled exercise studies and reported the number of low-trauma major osteoporotic fractures separately for exercise (EG) and control (CG) groups. Primary study outcome was incidence ratio (IR) for major osteoporotic fractures. Sub-analyses were conducted for progression of intensity (yes vs. no) during the trial and the study duration (≤ 12 months vs. > 12 months).
Results
In summary, 11 studies with a pooled number of 9715 participant-years in the EG and 9592 in the CG were included. The mixed-effects conditional Poisson regression revealed positive exercise effects on major osteoporotic fractures (RR: 0.75, 95% CI: 0.54–0.94, p = .006). Although studies with intensity progression were more favorable, our subgroup analysis did not determine significant differences for diverging intensity progression (p = .133) or study duration (p = .883). Heterogeneity among the trials of the subgroups (I2 ≤ 0–7.1%) was negligible.
Conclusion
The present systematic review and meta-analysis provided significant evidence for the favorable effect of exercise on major osteoporotic fractures. However, diverging study and exercise characteristics along with the close interaction of exercise parameters prevented the derivation of reliable recommendations for exercise protocols for fracture reductions.
PROSPERO ID: CRD42021250467.
Similar content being viewed by others
Introduction
Low-trauma fractures related to osteoporosis are a major problem in our aging society. Considering the demographic change in Europe, the number of osteoporotic fractures will quite likely increase by about 25% during the next 10–15 years [1]. A large variety of pharmaceutic agents target osteoporosis, most of which are very cost intensive, have potential negative adverse effects, and focus predominately on the bone. In contrast, physical exercise is a low-cost approach providing positive effects on fall risk [2] and bone strength [3, 4] without causing relevant adverse effects [5]. Thus, exercise might be an excellent strategy for combatting fractures in older adults. Reviewing the literature shows that there is indeed some evidence for a fracture-preventing effect of exercise in older adults [6,7,8,9]. However, with the exception of an older systematic review and meta-analysis that focused on low-trauma overall fractures [7], all the other studies [6, 8, 9] focused on data regarding fall-related fractures. In a recent systematic review and meta-analysis, we determined significant positive effects of exercise on overall and major osteoporotic fracture incidence [10]. Nevertheless, due to the considerable heterogeneity between the trial results, it is important to identify key components of promising exercise protocols. While their close interaction might prevent a meaningful sub-analysis of many exercise parameters (e.g., exercise intensity), we focus on intensity progression during the trial and study duration, as these may well be more independent training parameters. Thus, besides providing evidence for a (osteoporotic) fracture-preventing effect of exercise, we concentrated on the corresponding effect of (1) the progression of intensity during exercise intervention and (2) the duration of the study intervention,Footnote 1 in order to derive reliable exercise recommendations.
Methods
This systematic review and meta-analysis is part of the Austria/German/Swiss (DACH) S3 Guideline “körperliches Training zur Frakturprophylaxe” (AWMF: 183—002).
Literature search
We adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11]. Briefly, we checked six electronic databases (PubMed, Scopus, Web of Science, Cochrane, Science Direct, and ERIC) without language restrictions for articles published from January 1, 2013 (last search [7]) to May 2021. We applied keywords and their synonyms around the queries “Bone mass” or “Osteopenia” or “Bone turnover” or “Bone metabolism” or “Bone mineral content” or “Skeleton” or “Bone Mineral Density” or “BMD” or “Bone Density” or “Osteoporoses” or “Osteoporosis” or “Bone structure” or “Bone status” or “Bone Tissue” or “bone”) AND (“Bone fracture” or “Fracture” or “fragility fracture” or “Broken Bone”) AND (“Exercise” or “physical activity” or “Physical training” or “Exercise training”) AND (“clinical trial”) AND (“45 years and older”). We also checked reference lists of eligible studies and systematic reviews/meta-analysis that focused on fracture and fall reduction and bone-related outcomes (e.g., BMD). Studies without full texts were not considered.
Eligibility criteria
Briefly, randomized and non-randomized clinical studies were included that fulfilled the following eligibility criteria: (a) exercise studies on fracture prevention, fall reduction, and bone strength with (b) at least one exercise (EG) versus one control group (CG) that (c) reported the number of hip, lumbar spine, forearm, and/or humerus fractures (d) separately for EG and CG, independently of (e) whether fractures were defined as primary or secondary outcome, observation, or adverse event, and (e) female and male cohorts older than 50 years on average that were observed (f) for at least 3 months (i.e., study length ≥ 3 months).
Studies that supplied (a) pharmaceutic agents (e.g., glucocorticoids, bisphosphonates) or treatments (e.g., chemo- and/or radiotherapy) with relevant impact on bone metabolism, (b) trials/study groups with mixed interventions other than exercise and low-dosed calcium/cholecalciferol were excluded. We also excluded review articles, case reports, editorials, conference abstracts, letters, preliminary data, or duplicate studies. For the present subgroup analyses, studies (i.e., [12,13,14]) that terminated their intervention 6 months ago and longer were not considered.
Data extraction
During the first step, two reviewers (IH, MS) independently reviewed the titles and abstracts for eligible articles. Subsequently, full-text articles were reviewed by IH and WK. Eligible articles were extracted by IH and WK using a detailed extraction form that asked for study characteristics, study protocol, participant and exercise characteristics, supplementation, and fractures in the EG and CG. In the case of missing information, the authors in question were contacted (n = 6).
Outcome measures
As per FRAX [15], low-trauma fractures of the arm, forearm, or wrist and hip and vertebral fractures were summarized into major osteoporotic fracture as the primary study outcome of the present study. Fractures induced by falls from levels higher than standing and car or bicycle accidents were not included. However, in a minor variation from FRAX, all types of humerus and vertebral fractures were included.
Quality assessment
The Physiotherapy Evidence Database (PEDro) scale risk of bias tool [16] and the TESTEX (Tool for the assEssment of Study qualiTy and reporting in Exercise) score [17] specifically dedicated to evaluate the methodologic quality of physiotherapy and exercise trials was used to rate methodologic quality of the exercise trials.
Data synthesis
Of importance, we pooled the three different exercise groups of Karinkanta et al. [18] into one exercise group. With respect to the study of Bischoff-Ferrari et al. [19], we included the isolated exercise group (without vitamin D) with data provided by the authors. As stated, we focused on two research issues, intensity progression and duration of the exercise study in the sub-analyses. Two reviewers (IH, WK) independently categorized the trials into the subgroups, with full consensus for classification.
Statistical analysis
We used the mixed-effects conditional Poisson regression model suggested by Stijnen et al. [20] for our analysis. We applied R packages metafor [21] included in the statistical software R [22]. The incidences were transformed into incidence rate ratios (IR) along with 95% confidence intervals (95% CI). Heterogeneity between studies was checked using I2 statisticsFootnote 2 [23] in combination with a Wald and likelihood ratio test, respectively. Funnel plots with Kendall’s τ statistic were applied to explore potential small study/publication bias. Subgroup analyses were applied for subgroups as described in data synthesis above. All tests were 2-tailed, and significance was accepted at p < 0.05.
Results
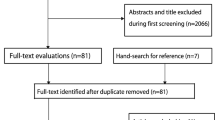
Our search identified 11 eligible studies [18, 19, 24,25,26,27,28,29,30,31,32] (Fig. 1) with a pooled number of participant years of n = 9715 in the EG and n = 9592 in CG. All studies included community dwelling middle-aged to older cohorts.
Flow-chart of the present systematic review according to PRISMA [11]
Table 1 gives a summary of the study and participant characteristics. In summary, no relevant between group differences (EG vs. CG) were observed for baseline participant characteristics of the individual studies. Initial sample sizes varied from 27 to 3279 participants/group. All but two studies [24, 32] included Caucasian cohorts on average between 54 ± 3 {Chan, 2004 #8453} and 80 ± 4 {Sakamoto, 2013 #15970} years of age. Seven studies focused exclusively on women. Six studies defined fracture risk as the primary outcome (Table 1).
Exercise characteristics
Table 2 displays exercise characteristics of the included studies. The exercise program of three studies focused predominately on combined fall prevention/bone strengthening [25, 27, 28] or fall prevention protocols [26, 30, 32], while four studies [18, 24, 29, 31] concentrated on bone strengthening only. Length of the exercise intervention ranged from 6 months [32] to 16 years [28]. Unfortunately, not all of the studies reported the exercise intensity applied for the respective training component adequately and comprehensively (Table 2). With respect to physical interventions in the control group, at least three studies [19, 25, 27] implemented an “active control group.”
Supplementation with vitamin D and/or calcium
Two studies [27, 28] provided calcium (up to 1000 mg/d) supplements for the EG and CG.
Methodological quality
The methodological quality is listed in Table 3. Score points applying PEDro vary between 3 and 9 from a maximum of 10 (9) points and 6–14 from a maximum of 15 when applying the TESTEX score. Of importance, blinding of instructors (i.e., treatment providers) is not applicable in exercise studies; consequently, the maximum score for PEDro should be considered 9 points. In contrast, TESTEX did not score blinding of treatment providers and participants.
Altogether we observed 151 major osteoporotic fractures (MOF) in the exercise and 196 fractures in the control group. Excluding the follow-up studies, 126 MOF were observed in the EG versus 162 MOF in the CG. In detail, 44 versus 58 hip fractures were recorded in the EG vs. CG; in parallel 62 (EG) vs. 52 (CG) forearm and wrist fractures were reported. Unfortunately, some studies did not report vertebral fractures; thus, the number of 25 fractures in the EG vs. 49 in the CG might be considerably underreported.
Meta-analysis results
The meta-analysis demonstrated a significant (p = 0.006) effect of exercise on major osteoporotic fractures (IR: 0.75; 95% CI: 0.59–0.94) (Figs. 2 and 3, lower part). Heterogeneity between the trials (I2 < 1%) was negligible, and funnel plots and tests for funnel plot asymmetry indicate no relevant evidence for publication/small study bias.
Subgroup analysis on exercise components
Progression of intensity during the exercise trial
Five studies provided intensity progression in their exercise protocols [18, 26,27,28,29], while another six studies did not change exercise intensity during the intervention (Table 2, Fig. 2). While we observed more favorable effects in the subgroups that applied progression of intensity, in summary, we did not determine a significant difference between the two subgroups (p = 0.133) (Fig. 2). Heterogeneity between the trials was negligible for both subgroups (I2 = 0% and 7.1%).
Duration of the intervention protocol of the exercise trial
Only three studies applied study protocols ≤ 12 months [18, 24, 32], while another eight studies exercised > 12 months to 16 years (Table 2, Fig. 3). In contrast to the shorter studies/exercise interventions (IR: 0.70; 95% CI: 0.23 to 2.15), we observed significant effects for the exercise trials of longer duration (IR: 0.77; 95% CI: 0.61 to 0.97); however, in summary, no relevant differences between the two subgroups (p = 0.883) (Fig. 3) were observed. Heterogeneity between the trials was negligible for both subgroups (I2 = 0%).
Discussion
Apart from generating evidence for the fracture-preventing effect of exercise on low-trauma, major osteoporotic fractures [15], the present systematic review and meta-analysis aimed to determine parameters that might explain the effectiveness of exercise in reducing fractures related to osteoporosis in middle-aged to older adults. In summary the study provided evidence for a significant (major osteoporotic) fracture reducing effect of exercise; however, the sub-analysis on the relevance of intensity progression and exercise duration on this positive interaction did not significantly support the relevance of these important exercise parameters/principles.
Due to the close interaction of exercise parameters [33] and the few exercise trials that focus on definite outcomes of fracture reduction, it is a daunting task to identify key (exercise) parameters for generating meaningful recommendations for promising exercise protocols. This refers especially to the area of fracture reduction with its fundamentally different training strategies on bone strengthening and/or fall reduction [34]. As most exercise parameters (e.g., type of exercise, exercise intensity, training frequency) were confounded by the aspects described above, we focused on the principle of (intensity) progression and the duration of the study intervention because these can be considered superordinate variables of exercise training protocols.
Progression, i.e., the frequent adaptation of training load to persistently apply the overload principle [33], can be realized by changing several parameters including exercise frequency and/or intensity, type of exercise, or exercise duration. However, with few exceptions [27, 28], most of the included exercise trials focused (if at all) on the progression of exercise intensity. In summary, we observed more favorable effects of studies that applied intensity progression (vs. non-progression) on major osteoporotic fracture numbers; nevertheless, differences between the subgroups remained non-significant (Fig. 2). One may argue that progression might be negligible in studies of short duration, but the only study to which this could applied is the 6-month study of Sakamoto et al. [32]. Another reason for our finding might be that progression in particular of balance protocols was rarely reported and the corresponding studies were thus not correctly classified by our approach.
Although this aspect is not negligible for fall prevention studies [2] either, it is outweighed by duration of the study/intervention in exercise programs on bone strengthening due to the length of bone adaptation in adults [35, 36]. Furthermore, along with high sample sizes, study duration is important for generating enough statistical power to address fracture number as a clinical outcome [28]. Of importance only three studies applied exercise protocols of 6 [32] to 12 months [18, 24]. Comparing the latter studies with longer studies (Fig. 3), we observed comparable effects sizes for the two categories.
In summary, we provided further evidence for the (osteoporotic) fracture-reducing effect of exercises; however, we failed to determine key parameters of promising exercise parameters or training principles in this area. We predominately attribute this unfavorable result to the fact that due to participant characteristics,Footnote 3 two fundamentally different exercise strategies, i.e., bone strengthening or fall reduction (or both), can be applied for reducing fracture risk. Considerable differences in addressing the two training aims confounded a joint analysis for meaningful exercise parameters and training principles. In parallel, against our expectation, with its close interaction of exercise parameters, the complexity of exercise might have also confounded our analysis of intensity progression and study duration. Since the methodologically correct approach for addressing this problem, i.e., trials with two exercise arms that differ only in the given component of interest (e.g., exercise frequency; [37]), was not availableFootnote 4 in the domain of fracture reduction, corresponding exercise recommendations have to be derived from more dedicated meta-analyses in the area of osteoporosis [38,39,40] or fall reduction [2, 41] or even better: from randomized controlled trials with similar or comparable training aims and cohorts.
Due to higher evidence standards, more dedicated inclusion criteria, higher fracture risk, and diverging fracture outcomes, it is difficult to set our results into perspective with data on pharmaceutic studies. However, a (very) rough overview on bisphosphonate (risedronate[42],Footnote 5 zoledronate [43], denosumab [44], and teriparatide [45]) effects on fragility fracture incidence indicates results in the area of 20% (risedronate, non-vertebral fractures) to 54% (teriparatide, overall fragility fractures). Our result falls at the lower range; however, it should be borne in mind that all of these pharmaceutic studies focus on secondary preventions, i.e., subjects with a much higher fracture prevention potential. Evidence for a fracture preventing effect in the area of primary prevention is much lower to negligible (e.g., [42]). From a socioeconomic point of view, on the other hand, it would be wrong to conclude that exercise might be a true alternative to pharmaceutical therapy. A large proportion of frail elderly persons, the most vulnerable group for fractures, demonstrate low affinity to exercise [46] and will be hard to persuade to start exercising frequently. Nevertheless, the combination of bone strengthening drugs and fall prevention exercise will definitely be the most promising fracture reduction strategy for this cohort.
Apart from problems described above, other limitations and/or particularities of the present work might have affected our results. (1) We focused on low-trauma fractures and thus excluded fractures caused, for example, by bicycle or car accidents, or falls from levels higher than standing. However, due to unavailable data, we might have not included only “low-trauma fractures.” However, considering that in osteoporosis not only fragility fractures but also all forms of fractures, including high-impact trauma fractures, occur quite frequently, this limitation might be negligible. (2) We further subsume all types of vertebral and humerus fractures under “major osteoporotic fractures,” which is not consistent with FRAX [15]. (3) We included studies with “active control groups” (Table 3) which might have diluted our exercise effects on fracture reduction slightly. (4) We included one non-randomized controlled trial [28]. Although fully aware of potential sources of bias,Footnote 6 we included this study due to its long duration (16 years), the sufficient power to address fracture as an outcome, and “fracture reduction” being stated as the primary outcome. (5) The latter aspect might be highly relevant since studies that focus on BMD effects (“bone-strength,” Table 3) in older people, for example, did not adequately address all the relevant fracture determinants and thus may have generated suboptimum results. (6) Heterogeneity between the trials was consistently negligible (i.e., I2: 0 to 7.1%) among the subgroups (Figs. 2 and 3). Further funnel plot analyses did not indicate evidence for publication/small-study bias. This finding is noteworthy because the studies vary widely with respect to participant (Table 1) and exercise characteristics (Table 2). (7) Finally, the statistical power to address differences between the subgroups can be considered moderate at best. Nevertheless, a recent (meta-)analysis on major osteoporotic fracture reduction that focused on supervision of the exercise program revealed significant differences in favor of supervised exercise protocols {Hoffmann, 2022 #16145}. Thus, the present analysis does not seem to be “hopelessly underpowered.”
Conclusion
Our systematic review and meta-analysis showed a 23% reduction in major osteoporotic fracture incidence, thus providing further evidence for the significant favorable effect of exercise on (low-trauma) fracture reduction. On the other hand, our joint analysis of exercise protocols that focus on bone strengthening, fall reduction, or both did not indicate high relevance of intensity progression or study duration. Along with others [47], we feel that meta-analyses might not be the best choice for deriving promising exercise recommendations, at least for the area of fracture reduction due to the complexity of exercise and the close interaction of exercise parameters. Well-designed and adequately powered randomized controlled trials might be more suitable to address this issue.
Data availability
The data that support the findings of this study are available from the corresponding author (WK), upon reasonable request.
Notes
More precisely, the studies listed the length of the intervention as “study duration.” Since no included study reported a delay between baseline or follow-up assessment and start or end of the intervention, we consistently use the term “study duration.”.
0–40%, low; 30–60%, moderate; 50–90%, substantial; 75–100%, considerable heterogeneity.
E.g., early-postmenopausal osteopenic women with high bone turnover and low risk of falls versus vulnerable older people with manifest osteoporosis, pharmaceutic therapy, and high fall risk.
Certainly due to the need to generate an enormous power (ie number of participant years) to address fracture number as an outcome considering further the potentially small differences between the groups….
In the area of secondary prevention; effects on primary prevention were much lower.
While we do not observe differences in prognostic outcome measures, adherence rates were higher compared to most comparable RCTs.
References
Kanis JA, Norton N, Harvey NC et al (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos 16:82. https://doi.org/10.1007/s11657-020-00871-9
Sherrington C, Michaleff ZA, Fairhall N et al (2017) Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med 51:1750–1758. https://doi.org/10.1136/bjsports-2016-096547
Shojaa N, von Stengel S, Schoene D et al (2020) Effect of exercise training on bone mineral density in postmenopausal women: a systematic review and meta-analysis of intervention studies. Front Physiol 11:1427–1444. https://doi.org/10.3389/fphys.2020.00652
Mages M, Shojaa M, Kohl M et al (2021) Exercise effects on bone mineral density in men. Nutrients 13:4244. https://doi.org/10.3390/nu13124244
Kunutsor SK, Leyland S, Skelton DA et al (2018) Adverse events and safety issues associated with physical activity and exercise for adults with osteoporosis and osteopenia: a systematic review of observational studies and an updated review of interventional studies. J Frailty Sarcopenia Falls 3:155–178. https://doi.org/10.22540/JFSF-03-155
de Souto BP, Rolland Y, Vellas B, Maltais M (2019) Association of long-term exercise training with risk of falls, fractures, hospitalizations, and mortality in older adults: a systematic review and meta-analysis. JAMA Intern Med 179:394–405. https://doi.org/10.1001/jamainternmed.2018.5406
Kemmler W, Haberle L, von Stengel S (2013) Effects of exercise on fracture reduction in older adults: a systematic review and meta-analysis. Osteoporos Int 24:1937–1950. https://doi.org/10.1007/s00198-012-2248-7
Wang Q, Jiang X, Shen Y et al (2020) Effectiveness of exercise intervention on fall-related fractures in older adults: a systematic review and meta-analysis of randomized controlled trials. BMC Geriatr 20:322. https://doi.org/10.1186/s12877-020-01721-6
Zhao R, Feng F, Wang X (2017) Exercise interventions and prevention of fall-related fractures in older people: a meta-analysis of randomized controlled trials. Int J Epidemiol 46:149–161. https://doi.org/10.1093/ije/dyw142
von Stengel S, Becker C, Gosch M, Jakob F, Kerschan-Schindl K, Kladny B, Clausen J, Lange U, Middeldorf S, Peters S, Schoene D, Sieber C, Tholen R, Thomasius F, Bischoff-Ferrari HA, Uder M, Kemmler W (2022) Exercise reduces the number of overall and major osteoporotic fractures in adults. Does supervision make a difference? Systematic review and meta-analysis. J Bone Miner Res. https://doi.org/10.1002/jbmr.4683. Online ahead of print
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
Karinkanta S, Kannus P, Uusi-Rasi K, Heinonen A, Sievanen H (2015) Combined resistance and balance-jumping exercise reduces older women’s injurious falls and fractures: 5-year follow-up study. Age Ageing 44:784–789. https://doi.org/10.1093/ageing/afv064
Korpelainen R, Keinanen-Kiukaanniemi S, Nieminen P, Heikkinen J, Vaananen K, Korpelainen J (2010) Long-term outcomes of exercise: follow-up of a randomized trial in older women with osteopenia. Arch Intern Med 170(17):1548–1556. https://doi.org/10.1001/archinternmed.2010.311
Sinaki M, Itoi E, Wahner HW et al (2002) Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone 30:836–841
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397. https://doi.org/10.1007/s00198-007-0543-5
Sherrington C, Herbert RD, Maher CG, Moseley AM (2000) PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther 5:223–226. https://doi.org/10.1054/math.2000.0372
Smart NA, Waldron M, Ismail H et al (2015) Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 13:9–18. https://doi.org/10.1097/XEB.0000000000000020
Karinkanta S, Heinonen A, Sievanen H et al (2007) A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int 18:453–462
Bischoff-Ferrari HA, Vellas B, Rizzoli R et al (2020) Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA 324:1855–1868. https://doi.org/10.1001/jama.2020.16909
Stijnen T, Hamza TH, Ozdemir P (2010) Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 29:3046–3067. https://doi.org/10.1002/sim.4040
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. J Stat Softw 36:1–48. https://doi.org/10.18637/jss.v036.i03
R-Core-Team (2021) R. A language and environment for statistical computing. In R Foundation for Statistical Computing (ed), stij edn. https://www.R-project.org/. Vienna, Austria
Higgins J, Thomas J, Chandler J et al (2021) Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane, 2021
Chan K, Qin L, Lau M et al (2004) A randomized, prospective study of the effects of Tai Chi Chun exercise on bone mineral density in postmenopausal women. Arch Phys Med Rehabil 85:717–722
Ebrahim SB, Thompson PW, Baskaran V, Evans K (1997) Randomized placebo controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age Aging 26:252–260
Gill TM, Pahor M, Guralnik JM et al (2016) Effect of structured physical activity on prevention of serious fall injuries in adults aged 70–89: randomized clinical trial (LIFE Study). BMJ 352:i245. https://doi.org/10.1136/bmj.i245
Kemmler W, von Stengel S, Engelke K, Haberle L, Kalender WA (2010) Exercise effects on bone mineral density, falls, coronary risk factors, and health care costs in older women: the randomized controlled senior fitness and prevention (SEFIP) study. Arch Intern Med 170(2):179–185. https://doi.org/10.1001/archinternmed.2009.499
Kemmler W, Bebenek M, Kohl M, Von Stengel S (2015) Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Osteoporos Int 26:2491–2499. https://doi.org/10.1007/s00198-015-3165-3
Korpelainen R, Keinanen-Kiukaanniemi S, Heikkinen J, Vaananen K, Korpelainen J (2006) Effect of exercise on extraskeletal risk factors for hip fractures in elderly women with low BMD: a population-based randomized controlled trial. J Bone Miner Res 21:772–779
Lamb SE, Bruce J, Hossain A et al (2020) Screening and intervention to prevent falls and fractures in older people. N Engl J Med 383:1848–1859. https://doi.org/10.1056/NEJMoa2001500
Preisinger E, Alacamlioglu Y, Pils K et al (1996) Exercise therapy for osteoporosis: results of a randomised controlled trial. Br J Sports Med 30:209–212
Sakamoto K, Endo N, Harada A et al (2013) Why not use your own body weight to prevent falls? A randomized, controlled trial of balance therapy to prevent falls and fractures for elderly people who can stand on one leg for </=15 s. J Orthop Sci 18:110–120. https://doi.org/10.1007/s00776-012-0328-3
Weineck J (2019) Optimales training. Spitta-Verlag, Erlangen
Kemmler W, Stengel V (eds) (2019) The role of exercise on fracture reduction and bone strengthening. Avademic Press, London
Erben RG (2015) Hypothesis: coupling between resorption and formation in cancellous bone remodeling is a mechanically controlled event. Front Endocrinol (Lausanne) 6:82. https://doi.org/10.3389/fendo.2015.00082
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 11:219–227. https://doi.org/10.1007/s11154-010-9153-1
Zitzmann AL, Shojaa M, Kast S et al (2021) The effect of different training frequency on bone mineral density in older adults. A comparative systematic review and meta-analysis. Bone 154:116230. https://doi.org/10.1016/j.bone.2021.116230
Beck BR, Daly RM, Singh MA, Taaffe DR (2016) Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J Sci Med Sport 20:438–445. https://doi.org/10.1016/j.jsams.2016.10.001
Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW (2019) Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther 23:170–180. https://doi.org/10.1016/j.bjpt.2018.11.011
Giangregorio LM, McGill S, Wark JD et al (2015) Too fit to fracture: outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporos Int 26:891–910. https://doi.org/10.1007/s00198-014-2881-4
Sibley KM, Thomas SM, Veroniki AA et al (2021) Comparative effectiveness of exercise interventions for preventing falls in older adults: a secondary analysis of a systematic review with network meta-analysis. Exp Gerontol 143:111151. https://doi.org/10.1016/j.exger.2020.111151
Wells GA, Hsieh SC, Zheng C, Peterson J, Tugwell P, Liu W (2022) Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 5:CD004523. https://doi.org/10.1002/14651858.CD004523.pub4
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822. https://doi.org/10.1056/NEJMoa067312
McCloskey EV, Johansson H, Oden A et al (2012) Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 27:1480–1486. https://doi.org/10.1002/jbmr.1606
Neer RM, Arnaud CD, Zanchetta JR (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Carlson SA, Fulton JE, Schoenborn CA, Loustalot F (2010) Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med 39:305–313. https://doi.org/10.1016/j.amepre.2010.06.006
Gentil P, Arruda A, Souza D et al (2017) Is there any practical application of meta-analytical results in strength training? Front Physiol 8:1. https://doi.org/10.3389/fphys.2017.00001
Acknowledgements
We acknowledge the support of the Elsbeth Bonhoff Stiftung, Berlin, Germany and the Dachverband Osteologie (DVO) e.V. We are also very grateful to all authors who provided missing information for the present work. The article was performed in (partial) fulfillment of the requirements for Isabelle Hoffmann’s obtaining the degree Dr. med. dent.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no external funding; however, the project S3-Guideline “körperliches Training zur Frakturprophylaxe” was supported by the Elsbeth Bonhoff Foundation.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed this systematic review and meta-analysis and drafted and revised the manuscript. Article search, screening, data extraction, and rating were performed by IH, MS, SvS, DS, HBF, and WK, and formal analysis was conducted by WK and MK. All authors read the final version of the manuscript. WK accepts direct responsibility for the work.
Corresponding author
Ethics declarations
Statement of human rights
This article does not cover any studies with human participants or animals performed by any of the authors.
Conflicts of interest
None.
Informed consent
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hoffmann, I., Kohl, M., von Stengel, S. et al. Exercise and the prevention of major osteoporotic fractures in adults: a systematic review and meta-analysis with special emphasis on intensity progression and study duration. Osteoporos Int 34, 15–28 (2023). https://doi.org/10.1007/s00198-022-06592-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06592-8