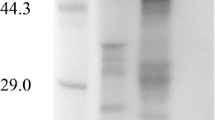
Abstract
Four aryl-phospho-β-d-glucosidases were identified in Bacillus subtilis by using 4-methylumbelliferyl-phospho-β-d-glucopyranoside as a substrate. Two of these enzymes are the products of the bglA and bglH genes, previously suggested to encode aryl-phospho-β-d-glucosidases, while the other enzymes are encoded by the yckE and ydhP genes. Together, these four genes account for >99.9% of the glucosidase activity in B. subtilis on aryl-phospho-β-d-glucosides. yckE was expressed at a low and constant level during growth, sporulation, and spore germination, and was not induced by aryl-β-d-glucosides. ydhP was also not induced by aryl-β-d-glucosides. However, while ydhP was expressed at only a very low level in exponential-phase cells and germinating spores, this gene was expressed at a higher levels upon entry into the stationary phase of growth. Strains lacking yckE or ydhP exhibited no defects in growth, sporulation, or spore germination or in growth on aryl-β-d-glucosides. However, a strain lacking bglA, bglH and yckE grew poorly if at all on aryl-β-d-glucosides as the sole carbon source.




Similar content being viewed by others
Abbreviations
- MU :
-
4-Methylumbelliferone
- MUG :
-
4-Methylumbelliferyl-β-d-glucopyranoside
- MUGal :
-
4-Methylumbelliferyl-β-d-galactopyranoside
- MUG-P :
-
4-Methylumbelliferyl-β-d-glucopyranoside-6-phosphate
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81:74–76
Davis BJ (1964) Disc electrophoresis. II. Methods and application to human serum proteins. Ann NY Acad Sci 121:404–407
Deutscher J, Galinier A, Martin-Verstraete I (2002) Carbohydrate uptake and metabolism. In: Hoch JA, Losick R, Sonenshein AL (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, DC, pp 129–150
Ferrari FA, Nguyen A, Long D, Hoch JA (1983) Construction of an integrable plasmid for Bacillus subtilis. J Bacteriol 154:1513–1515
Ferrari E, Howard SM, Hoch JA (1985) Effect of sporulation mutations on subtilisin expression, assayed using a subtilisin-β-galactosidase gene fusion. In: Hoch JA, Setlow P (eds) Molecular biology of microbial differentiation. American Society for Microbiology, Washington, DC, pp 180–184
Guerot-Fleury AM, Shazand K, Frandsen N, Stragier P (1995) Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336
Krüger S, Hecker M (1995) Regulation of the putative bglPH operon for aryl-β-glucoside utilization in Bacillus subtilis. J Bacteriol 177:5590–5597
Krüger S, Hecker M (1996) Transcriptional analysis of bglPH expression in Bacillus subtilis: evidence for two distinct pathways mediating catabolite repression. J Bacteriol 178:2637–2644
Le Coq D, Lindner C, Krüger S, Steinmetz M, Stülke J (1995) New β-glucoside (bgl) genes in Bacillus subtilis: the bglP gene product has both transport and regulatory functions similar to those of BglF, its Escherichia coli homolog. J Bacteriol 177:1527-1535
LeDeaux JR, Grossman AD (1995) Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J Bacteriol 177:166–175
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mason JM, Hackett RH, Setlow P (1988) Studies on the regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores using lacZ gene fusions. J Bacteriol 170:239–244
Miller J (1972) Assay of β-galactosidase. In: Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp 352–355
Nicholson WL, Setlow P (1990) Sporulation, germination and outgrowth. In: Harwood CR Cutting SM (eds) Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, England, pp 391–450
Paidhungat M, Setlow B, Driks A, Setlow P (2000) Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512
Paidhungat M, Setlow P (2002) Spore germination and outgrowth. In: Hoch JA, Losick R, Sonenshein AL (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, DC, pp 537–548
Rotman Y, Fields ML (1967) A modified reagent for dipicolinic acid analysis. Anal Biochem 22:168
Saier MH Jr, Goodman SR, Maile RR, Moreno MS, Weyler W, Yang N, Paulsen IT (2002) Overview of transport in Bacillus subtilis. In: Hoch JA, Losick R, Sonenshein AL (eds) Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, DC, pp 113–128
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Spizizen J (1958) Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA 44:1072–1078
Stragier P, Bonamy C, Karmazyn-Campelli C (1988) Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52:697–704
Thompson J, Pikis A, Ruvinov SB, Henrissat B, Yamamoto H, Sekiguchi J (1998) The gene glvA of Bacillus subtilis 168 encodes a metal-requiring NAD(H)-dependent 6-α-phosphoglucosidase. Assignment to family 4 of the glycosylhydrolase superfamily. J Biol Chem 273:27347–27356
Tobisch S, Glaser P, Krüger S, Hecker M (1997) Identification and characterization of a new β-glucoside utilization system in Bacillus subtilis. J Bacteriol 179:496–506
Wilson G, Fox CF (1974) The β-glucoside system of Eschericia coli. IV. Purification and properties of phospho-β-glucosidases A and B. J Biol Chem 249:5586–5598
Zhang, J, Aronson A (1994) A Bacillus subtilis bglA gene encoding phospho-β-glucosidase is inducible and closely linked to a NADH dehydrogenase-encoding gene. Gene 140:85–90
Acknowledgements
This work was supported by contracts from the 3M Corporation. We are grateful to A. Aronson for the gift of strain JZ30 and to Kasia Koziol-Dube for assistance with some experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Setlow, B., Cabrera-Hernandez, A., Cabrera-Martinez, R.M. et al. Identification of aryl-phospho-β-d-glucosidases in Bacillus subtilis . Arch Microbiol 181, 60–67 (2004). https://doi.org/10.1007/s00203-003-0628-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-003-0628-2