Abstract
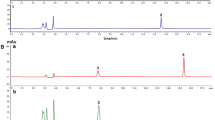
Two intestinal bacterial strains MT4s-5 and MT42 involved in the degradation of (−)-epigallocatechin (EGC) were isolated from rat feces. Strain MT4s-5 was tentatively identified as Adlercreutzia equolifaciens. This strain converted EGC into not only 1-(3, 4, 5-trihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (1), but also 1-(3, 5-dihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (2), and 4′-dehydroxylated EGC (7). Type strain (JCM 9979) of Eggerthella lenta was also found to convert EGC into 1. Strain MT42 was identified as Flavonifractor plautii and converted 1 into 4-hydroxy-5-(3, 4, 5-trihydroxyphenyl)valeric acid (3) and 5-(3, 4, 5-trihydroxyphenyl)-γ-valerolactone (4) simultaneously. Strain MT42 also converted 2 into 4-hydroxy-5-(3, 5-dihydroxyphenyl)valeric acid (5), and 5-(3, 5-dihydroxyphenyl)-γ-valerolactone (6). Furthermore, F. plautii strains ATCC 29863 and ATCC 49531 were found to catalyze the same reactions as strain MT42. Interestingly, formation of 2 from EGC by strain MT4s-5 occurred rapidly in the presence of hydrogen supplied by syntrophic bacteria. Strain JCM 9979 also formed 2 in the presence of the hydrogen or formate. Strain MT4s-5 converted 1, 3, and 4 to 2, 5, and 6, respectively, and the conversion was stimulated by hydrogen, whereas strain JCM 9979 could catalyze the conversion only in the presence of hydrogen or formate. On the basis of the above results together with previous reports, the principal metabolic pathway of EGC and EGCg by catechin-degrading bacteria in gut tract is proposed.







Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bolca S, Verstraete W (2010) Microbial equol production attenuates colonic methanogenesis and sulphidogenesis in vitro. Anaerobe 16:247–252
Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W (2005) Isolation and characterisation of an equal-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol 183:45–55
Goto K, Kanaya S, Nishikawa T, Hara H, Terada A, Ishigami T, Hara Y (1998) The influence of tea catechins on fecal flora of elderly residents in long-term care facilities. Ann Long-Term Care 6:43–48
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Higdon JV, Frei B (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 43:89–143
Ito K, Ishida H, Takagaki A, Nanjo F, Maruo T, Ito C (2008) Metabolism of isoflavone from soy bean by Eggerthella sp. MT4s-5, part 1 Metabolism of daidzein, glycitein, and genistein. The 128th Annual Meeting of the Pharmaceutical Society of Japan, Abstract 2, p 87
Jin J-S, Hattori M (2012) Isolation and characterization of a human intestinal bacterium Eggerthella sp. CAT-1 capable of cleaving the C-ring of (+)-catechin and (−)-epicatechin, followed by p-dehydroxylation of the B-ring. Biol Pharm Bull 35:2252–2256
Jin J-S, Zhao Y-F, Nakamura N, Akao T, Kakiuchi N, Min B-S, Hattori M (2007) Enantioselective dehydroxylation of enterodiol and enterolactone precursors by human intestinal bacteria. Biol Pharm Bull 30:2113–2119
Johnson R, Bryant S, Huntley AL (2012) Green tea and green tea catechin extracts: an overview of the clinical evidence. Maturitas 73:280–287
Kida K, Suzuki M, Matsumoto N, Nanjo F, Hara Y (2000) Identification of biliary metabolites of (−)-epigallocatechin gallate in rats. J Agric Food Chem 48:4151–4155
Kohri T, Matsumoto N, Yamakawa M, Suzuki M, Nanjo F, Hara Y, Oku N (2001) Metabolic fate of (−)-[4-3H] epigallocatechin gallate in rats after oral administration. J Agric Food Chem 49:4102–4112
Krumholz LR, Bryant MP (1986) Eubacterium oxidoreducens sp. nov. requiring H2 or formate to degrade gallate, pyrogallol, phloroglucinol and quercetin. Arch Microbiol 144:8–14
Kuroda Y, Hara Y (2004) Health effects of tea and its catechins. Kluwer Academic/Plenum Publishers, New York
Kutschera M, Engst W, Blaut M, Braune A (2011) Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol 111:165–175
Lee M-J, Wang Z-Y, Li H, Chen L, Sun Y, Gobbo S, Balentine DA, Yang CS (1995) Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev 4:393–399
Maruo T, Sakamoto M, Ito C, Toda T, Benno Y (2008) Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol 58:1221–1227
Minamida K, Ota K, Nishimukai M, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K (2008) Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int J Syst Evol Microbiol 58:1238–1240
Ohno M, Okano I, Watsuji T, Kakinuma T, Ueda K, Beppu T (1999) Establishing the independent culture of a strictly symbiotic bacterium Symbiobacterium thermophilum from its supporting Bacillus strain. Biosci Biotechnol Biochem 63:1083–1090
Sakamoto M, Takagaki A, Matsumoto K, Kato Y, Goto K, Benno Y (2009) Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family ‘Porphyromonadaceae’ isolated from rat faeces. Int J Syst Evol Microbiol 59:1748–1753
Sang S, Lee M-J, Yang I, Buckley B, Yang CS (2008) Human urinary metabolite profile of tea polyphenols analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry with data-dependent acquisition. Rapid Commun Mass Spectrom 22:1567–1578
Stams AJM, Plugge CM (2009) Electron transfer in syntrophic communication of anaerobic bacteria and archaea. Nat Rev Microbiol 7:568–577
Stams AJM, Bok FAM, Plugge CM, Eekert MHA, Dolfing J, Schraa G (2006) Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol 8:371–382
Takagaki A, Nanjo F (2010) Metabolism of (−)-epigallocatechin gallate by rat intestinal flora. J Agric Food Chem 58:1313–1321
Takagaki A, Otani S, Nanjo F (2011) Antioxidative activity of microbial metabolites of (−)-epigallocatechin gallate produced in rat intestines. Biosci Biotechnol Biochem 75:582–585
Thawornkuno C, Tanaka M, Sone T, Asano K (2009) Biotransformation of daidzein to equol by crude enzyme from Asaccharobacter celatus AHU 1763 required an anaerobic environment. Biosci Biotechnol Biochem 73:1435–1438
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wang L-Q, Meselhy MR, Li Y, Nakamura N, Min B-S, Qin G-W, Hattori M (2001) The heterocyclic ring fission and dehydroxylation of catechins and related compounds by Eubacterium sp. strain SDG-2, a human intestinal bacterium. Chem Pharm Bull 49:1640–1643
Yang CS, Wang X (2010) Green tea and cancer prevention. Nutr Cancer 62:931–937
Acknowledgments
We thank Matsumoto K. in our laboratories for analysis of 16S rRNA gene sequences of bacterial isolates. And we acknowledge the assistance of Andrea K. Suzuki (Mitsui Norin Co. Ltd.) in the review of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Takagaki, A., Kato, Y. & Nanjo, F. Isolation and characterization of rat intestinal bacteria involved in biotransformation of (−)-epigallocatechin. Arch Microbiol 196, 681–695 (2014). https://doi.org/10.1007/s00203-014-1006-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1006-y