Abstract.
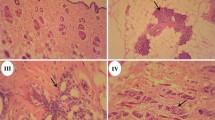
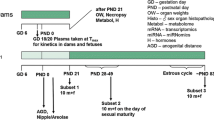
We performed a 28-day repeated-dose toxicity study of ethynylestradiol (EE) and bisphenol A (BPA) based on the draft protocol of the 'Enhanced OECD Test Guideline 407', and assessed the sensitivity of a list of parameters for detecting endocrine-related effects of endocrine disruption. Doses of EE at 0, 10, 50 or 200 µg/kg per day, or BPA at 0, 40, 200 or 1000 mg/kg per day were orally administered to Sprague-Dawley rats. The highest dose of BPA was decreased to 600 mg/kg per day from the second week of administration because a male rat given 1000 mg/kg BPA had died within 1 week with toxic clinical signs. In the assay using EE, the decrease of prostate, seminal vesicle and pituitary weights, increase of the testis weight, atrophic changes of the prostate, seminal vesicle and mammary gland, and degenerative changes in the testes were detected in male rats in the 50 and/or 200 µg/kg groups. In females of the 200 µg/kg group, decrease of the ovary weight, increase of the uterine weight, atrophy of the ovary, hypertrophy or squamous metaplasia of the uterine epithelial cells and mucification in the vagina were observed. Furthermore, diestrous, estrous or the unknown stage was prolonged in the 50 and 200 µg/kg groups of rats. Endocrine-mediated effects of EE were not detected in general observations, hematology, serum biochemistry, or hormonal or spermatological examinations. In the assay using BPA, the diestrous stages were prolonged at the highest dose, but changes related to endocrine effects were not detected in other examinations. Thus, among the parameters tested, the weight of endocrine-linked organs and their histopathological assessment and estrous cycle stage allowed the detection of the endocrine-related effect of EE, whereas the estrous cycle stage was only a useful parameter to detect the effect of BPA.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Yamasaki, K., Sawaki, M., Noda, S. et al. Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the 'Enhanced OECD Test Guideline no. 407'. Arch Toxicol 76, 65–74 (2002). https://doi.org/10.1007/s00204-001-0319-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00204-001-0319-1