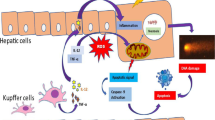
Abstract
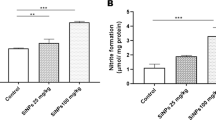
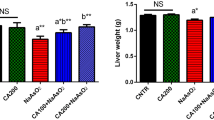
Cytokeratins (CK) constitute a family of cytoskeletal intermediate filament proteins that are typically expressed in epithelial cells. An abnormal structure and function are effects that are clearly related to liver diseases as non-alcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma. We have previously observed that sodium arsenite (SA) induced the synthesis of CK18 protein and promotes a dose-related disruption of cytoplasmic CK18 filaments in a human hepatic cell line. Both abnormal gene expression and disturbance of structural organization are toxic effects that are likely to cause liver disease by interfering with normal hepatocyte function. To investigate if a disruption in the CK18 expression pattern is associated with arsenite liver damage, we investigated CK18 mRNA and protein levels in liver slices treated with low levels of SA. Organotypic cultures were incubated with 0.01, 1 and 10 μM of SA in the absence and presence of N-acetyl cysteine (NAC). Cell viability and inorganic arsenic metabolism were determined. Increased expression of CK18 was observed after exposure to SA. The addition of NAC impeded the oxidative effects of SA exposure, decreasing the production of thiobarbituric acid-reactive substances and significantly diminishing the up regulation of CK18 mRNA and protein. Liver arsenic levels correlated with increased levels of mRNA. Mice treated with intragastric single doses of 2.5 and 5 mg/kg of SA showed an increased expression of CK18. Results suggest that CK18 expression may be a sensible early biomarker of oxidative stress and damage induced by arsenite in vitro and in vivo. Then, during SA exposure, altered CK expression may compromise liver function.





Similar content being viewed by others
References
ATSDR (2000) Toxicological profile for arsenic. Agency for Toxic Substances and Disease Registry, Atlanta
Azri S, Gandolfi AJ, Brendel K (1990) Carbon tetrachloride toxicity in precision cut tissue slices. In vitro Toxicol 3:127–138
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cadrin M, Hovington H, Marceau N, McFarlane-Anderson N (2000) Early perturbations in keratin and actin gene expression and fibrillar organisation in griseofulvin-fed mouse liver. J Hepatol 33:199–207
Calnek D, Quaroni A (1993) Differential localization by in situ hybridization of distinct keratin mRNA species during intestinal epithelial cell development and differentiation. Differentiation 53:95–104
Caulin C, Ware CF, Magin TM, Oshima RG (2000) Keratin dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol 149:17–22
Centeno JA, Mullick FG, Martinez L, Page NP, Gibb H, Longfellow D, Thompson C, Ladich ER (2002) Pathology related to chronic arsenic exposure. Environ Health Perspect 110:883–886
Chen YH, Wang JP, Wang H, Sun MF, Wei LZ, Wei W, Xu DX (2005) Lipopolysaccharide treatment downregulates the expression of the pregnane X receptor, cyp3a11 and mdr1a genes in mouse placenta. Toxicology 211:242–252
Coulombe PA, Omary MB (2002) ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 14:110–122
De Flora S, Izzotti A, D’Agostini F, Balansky RM (2001) Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis 22:999–1013
Denk H, Stumptner C, Zatloukal K (2000) Mallory bodies revisited. J Hepatol 32:689–702
Fickert P, Trauner M, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H (2002) Cytokeratins as targets for bile acid-induced toxicity. Am J Pathol 160:491–499
Gilbert S, Loranger A, Daigle N, Marceau N (2001) Simple epithelium keratins 8 and 8 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J Cell Biol 154:763–773
Han SG, Castranova V, Vallyathan V (2005) Heat shock protein 70 as an indicator of early lung injury caused by exposure to arsenic. Mol Cell Biochem 277:153–164
Hansen JM, Zhang H, Jones DP (2006) Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal Ions. Free Radic Biol Med 40:138–145
Hirano S, Kobayashi Y, Cui X, Kanno S, Hayakawa T, Shraim A (2004) The accumulation and toxicity of methylated arsenicals in endothelial cells: important roles of thiol compounds. Toxicol Appl Pharmacol 198:458–467
Hughes MF, Kitchin KT (2006) Arsenic, oxidative stress and carcinogenesis. In: Oxidative stress. Disease and cancer, chapter 29
Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Del Razo LM, Thomas DJ (2003) Accumulation and metabolism of arsenic in mice after repeated oral administration of arsenate. Toxicol Appl Pharmacol 191:202–210
IPCS/WHO (2001) IPCS (International Programme on Chemical Safety) Environmental Health Criteria 224. Arsenic and arsenic compounds, 2nd edn. World Health Organization, Geneva
Izquierdo-Vega JA, Soto CA, Sanchez-Peña LC, De Vizcaya-Ruiz A, Del Razo LM (2006) Diabetogenic effects and pancreatic oxidative damage in rats subchronically exposed to arsenite. Toxicol Lett 160:135–142
Kelly GS (1998) Clinical applications of N-acetylcysteine. Altern Med Rev 3:114–127
Kim YH, Park EJ, Han ST, Park JW, Kwon TK (2005) Arsenic trioxide induces Hsp70 expression via reactive oxygen species and JNK pathway in MDA231 cells. Life Sci 77:2783–2793
Kitchin KT, Ahmad S (2003) Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett 137:3–13
Ku NO, Michie SA, Soetikno RM, Resurrección EZ, Broome RL, Omary MB (1998) Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J Cell Bio 143:2023–2032
Ku NO, Darling JM, Krams SM, Esquivel CO, Keeffe EB, Sibley RK (2003) Keratin 8 and 18 mutations are risk factors for developing liver disease of multiple etiologies. Proc Natl Acad Sci USA 100:6063–6068
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 277:680–685
Liao J, Lowthert LA, Ku NO, Fernandez R, Omary MB (1995) Dynamics of human keratin 18 phosphorylation: polarized distribution of phosphorylated keratins in simple epithelial tissues. J Cell Biol 131:291–1301
Liu J, Xie Y, Ward JM, Diwan BA, Waalkes MP (2004) Toxicogenomics analysis of aberrant gene expression in liver tumors and non tumorous livers of adult mice exposed in utero to inorganic arsenic. Toxicol Sci 77:249–257
Moll R (1993) Cytokeratins as markers of differentiation. Expression profiles in epithelia and epithelial tumors. Veroff Pathol 142:1–197
Moll R, Franke W, Schiller D, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Omary MB, Coulombe PA, McLean WH (2004) Intermediate filament proteins and their associated diseases. N Engl J Med 351:2087–100
Patrick L (2003) Toxic metals and antioxidants: part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev 8:106–128
Ramírez P, Del Razo LM, Gutierrez-Ruíz MC, Gonsebatt ME (2000) Arsenite induces DNA–protein crosslinks and cytokeratin expression in the WRL-68 human hepatic cell line. Carcinogenesis 21:701–706
Rhodes K, Oshima RG (1998) A regulatory element of the human keratin 18 gene with AP-1-dependent promoter activity. J Biol Chem 273:26534–26542
Santra A, Chowdhury A, Ghatak S, Biswas A, Dhali GK (2007) Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by n-acetylcysteine. Toxicol Appl Pharm. doi:10.1016/j.taap.2006.12.029
Sudheer AR, Muthukumaran S, Devipriya N, Menon VP (2007) Ellagic acid, a natural polyphenol protects rat peripheral blood lymphocytes against nicotine-induced cellular and DNA damage in vitro: with the comparison of N-acetylcysteine. Toxicology 230:11–21
Tao GZ, Toivola DM, Zhong B, Michie SA, Resurreccion EZ, Tamai Y, Taketo MM, Omary MB (2003) Keratin-8 null mice have different gallbladder and liver susceptibility to lithogenic diet-induced injury. J Cell Sci 116:4629–4638
Thomas DJ, Waters SB, Styblo M (2004) Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol 198:319–326
Wei M, Arnold L, Cano M, Cohen SM (2005) Effects of co-administration of antioxidants and arsenicals on the rat urinary bladder epithelium. Toxicol Sci 83:237–45
Yamanaka K, Okada S (1994) Induction of lung-specific DNA-damage by metabolically methylated arsenics via the production of free-radicals. Environ Health Perspect 102:37–40
Zatloukal K, Stumptner C, Lehner M, Denk H, Baribault H, Eshkind LG, Franke WW (2000) Cytokeratin 8 protects from hepatotoxicity, and its ratio to cytokeratin 18 determines the ability of hepatocytes to form Mallory bodies. Am J Pathol 156:1263–1274
Zhong B, Zhou Q, Toivola DM, Tao GZ, Resurrección EZ, Omary MB (2003) Keratin-8 null mice have different gallbladder and liver susceptibility to lithogenic diet-induced injury. J Cell Sci 116:4629–4638
Acknowledgments
This work was partially supported by PAPIIT IN203405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonsebatt, M.E., Del Razo, L.M., Cerbon, M.A. et al. Arsenite induced oxidative damage in mouse liver is associated with increased cytokeratin 18 expression. Arch Toxicol 81, 619–626 (2007). https://doi.org/10.1007/s00204-007-0192-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0192-7