Abstract
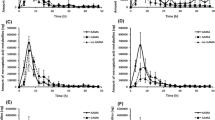
A dose of 0.99 mg d3-acrylamide (d3-AA) (13.2 μg/kg body weight) was ingested by a healthy male volunteer. Urine samples were collected over a period of 46 h after the intake and analyzed for the hydrolysis product of glycidamide (GA), 2,3-dihydroxy-propionamide (OH-PA), a metabolite of the toxicologically relevant oxidative AA metabolism pathway; 5.4% of the administered d3-AA dose was eliminated as OH-PA within 46 h after ingestion. Therefore, OH-PA represents a major metabolite of the oxidative metabolism pathway. Elimination kinetics of OH-PA is similar to the oxidative metabolites N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-cysteine (GAMA) and N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-cysteine (iso-GAMA). The major excretion of d3-OH-PA took place between 8 and 22 h with the highest urinary d3-OH-PA concentration (c max) of 69.3 μg/L urine, 18 h (t max) postdose. OH-PA (5.4%), together with the other known urinary metabolites of the oxidative pathway GAMA (4.6%) and iso-GAMA (0.8%), represents 10.8% of the total AA dose. The share of the oxidative pathway metabolites is much smaller than the share of the reductive pathway metabolite N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA) that represents 51.7% of the ingested d3-AA dose. However, this new quantitative human data on OH-PA together with the previous data on the other oxidative pathway metabolites are of special importance when evaluating the carcinogenic potential of AA and when comparing human data with data from animal studies.





Similar content being viewed by others
References
Bergmark E, Calleman CJ, Costa LG (1991) Formation of hemoglobin adducts of acrylamide and its epoxide metabolite glycidamide in the rat. Toxicol Appl Pharmacol 111:352–363
BgVV, Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin (2002) Statement of the BgVV. Einfluss der Ernährung auf die Aufnahme von Acrylamid
Boettcher MI, Bolt HM, Drexler H, Angerer J (2006) Excretion of mercapturic acids of acrylamide and glycidamide in human urine after single oral administration of deuterium-labelled acrylamide. Arch Toxicol 80:55–61
Calleman CJ, Bergmark E, Costa LG (1990) Acrylamide is metabolized to glycidamide in the rat: evidence from hemoglobin adduct formation. Chem Res Toxicol 3:406–412
Dearfield KL, Douglas GR, Ehling UH, Moore MM, Sega GA, Brusick DJ (1995) Acrylamide: a review of its genotoxicity and an assessment of heritable genetic risk. Mutat Res 330:71–99
DFG, Deutsche Forschungsgemeinschaft (2002) Maximale Arbeitsplatzkonzentrationen und biologische Arbeitsstoff Toleranzwerte 2002. Mitteilung 38 der Senatskommission zur Prüfung gesundheitsschädlicher Arbeitsstoffe
Doroshyenko O, Fuhr U, Kunz D, Frank D, Kinzig M, Jetter A, Reith Y, Lazar A, Taubert D, Kirchheiner J, Baum M, Eisenbrand G, Berger FI, Bertow D, Berkessel A, Sorgel F, Schomig E, Tomalik-Scharte D (2009) In vivo role of cytochrome P450 2E1 and glutathione-S-transferase activity for acrylamide toxicokinetics in humans. Cancer Epidemiol Biomarkers Prev 18:433–443
EPA US, Office of Toxic substances; US Environmental Protection Agency (1994) Chemical summary for acrylamide
EU, European Union, European Commission, Scientific Committee on Food (2001) Opinion on the results of the risk assessment of: acrylamide
Fennell TR, Sumner SC, Snyder RW, Burgess J, Spicer R, Bridson WE, Friedman MA (2005) Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol Sci 85:447–459
Fennell TR, Sumner SC, Snyder RW, Burgess J, Friedman MA (2006) Kinetics of elimination of urinary metabolites of acrylamide in humans. Toxicol Sci 93:256–267
Fuhr U, Boettcher MI, Kinzig-Schippers M, Weyer A, Jetter A, Lazar A, Taubert D, Tomalik-Scharte D, Pournara P, Jakob V, Harlfinger S, Klaassen T, Berkessel A, Angerer J, Sorgel F, Schomig E (2006) Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemiol.Biomarkers Prev 15:266–271
Gamboa da CG, Churchwell MI, Hamilton LP, Von Tungeln LS, Beland FA, Marques MM, Doerge DR (2003) DNA adduct formation from acrylamide via conversion to glycidamide in adult and neonatal mice. Chem Res Toxicol 16:1328–1337
Hartmann EC, Boettcher MI, Schettgen T, Fromme H, Drexler H, Angerer J (2008) Hemoglobin adducts and mercapturic acid excretion of acrylamide and glycidamide in one study population. J Agric Food Chem 56:6061–6068
Hartmann EC, Boettcher MI, Bolt HM, Drexler H, Angerer J (2009) N-Acetyl-S-(1-carbamoyl-2-hydroxy-ethyl)-L-cysteine (iso-GAMA) a further product of human metabolism of acrylamide: comparison with the simultaneously excreted other mercapturic acids. Arch Toxicol 83:731–734
IARC, International Agency for Research on Cancer (1994) Summary of Data Reported and Evaluation: acrylamide http://www.monographs.iarc.fr/ENG/Monographs/vol60/volume60.pdf. Accessed 7 July 2010
JRC-IRMM, Joint Research Center (JRC), Institute for Reference Materials and Measurements (IRMM), European Commission (2006) Monitoring Database Acrylamide http://www.irmm.jrc.ec.europa.eu/html/activities/acrylamide/EUacrylamidelevelmonitoringdatabase__statusJune2006.xls. Accessed 7 Jul 2010
Kopp EK, Dekant W (2009) Toxicokinetics of acrylamide in rats and humans following single oral administration of low doses. Toxicol Appl Pharmacol 235:135–142
Larsen K (1972) Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41:209–217
Miller MJ, Carter DE, Sipes IG (1982) Pharmacokinetics of acrylamide in Fisher-344 rats. Toxicol Appl Pharmacol 63:36–44
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419:448–449
Perez HL, Cheong HK, Yang JS, Osterman-Golkar S (1999) Simultaneous analysis of hemoglobin adducts of acrylamide and glycidamide by gas chromatography-mass spectrometry. Anal Biochem 274:59–68
Schettgen T, Broding HC, Angerer J, Drexler H (2002) Hemoglobin adducts of ethylene oxide, propylene oxide, acrylonitrile and acrylamide-biomarkers in occupational and environmental medicine. Toxicol Lett 134:65–70
Segerback D, Calleman CJ, Schroeder JL, Costa LG, Faustman EM (1995) Formation of N-7-(2-carbamoyl-2-hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis 16:1161–1165
Smith CJ, Perfetti TA, Rumple MA, Rodgman A, Doolittle DJ (2000) “IARC group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol 38:371–383
Sumner SC, MacNeela JP, Fennell TR (1992) Characterization and quantitation of urinary metabolites of [1, 2, 3-13C]acrylamide in rats and mice using 13C nuclear magnetic resonance spectroscopy. Chem Res Toxicol 5:81–89
Sumner SC, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI (1999) Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem Res Toxicol 12:1110–1116
Sumner SC, Williams CC, Snyder RW, Krol WL, Asgharian B, Fennell TR (2003) Acrylamide: a comparison of metabolism and hemoglobin adducts in rodents following dermal, intraperitoneal, oral, or inhalation exposure. Toxicol Sci 75:260–270
Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
WHO, World Health Organization (2002) Health implications of acrylamide in the food report of a Joint FAO/WHO consultation
Acknowledgments
We especially thank the DFG (German Research Foundation) for their financial support of the project (AN 107/17-1 and 17-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartmann, E.C., Latzin, J.M., Schindler, B.K. et al. Excretion of 2,3-dihydroxy-propionamide (OH-PA), the hydrolysis product of glycidamide, in human urine after single oral dose of deuterium-labeled acrylamide. Arch Toxicol 85, 601–606 (2011). https://doi.org/10.1007/s00204-010-0605-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-010-0605-x