Abstract
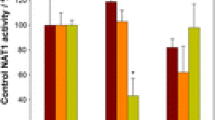
The potential of three isothiocyanates, namely R,S-sulforaphane, erucin and phenethyl isothiocyanate, of two naturally occurring glucosinolates, namely glucoerucin and glucoraphanin, and of the enantiomers of sulforaphane to modulate glucuronosyl transferase and epoxide hydrolase, two major carcinogen-metabolising enzyme systems, was investigated in precision-cut rat liver slices. Following exposure of the slices to the isothiocyanates (0–25 μM), erucin and phenethyl isothiocyanate, but not R,S-sulforaphane, elevated glucuronosyl transferase and epoxide hydrolase activities and expression, determined immunologically. Of the two enantiomers of sulforaphane, the R-enantiomer enhanced, whereas the S-enantiomer impaired, glucuronosyl transferase activity and only the former increased protein expression; furthermore, R-sulforaphane was more effective than the S-enantiomer in up-regulating microsomal epoxide hydrolase. When precision-cut rat liver slices were exposed to the same concentrations of glucoerucin and glucoraphanin, both glucosinolates caused a marked increase in the activity and expression of the microsomal epoxide hydrolase but had no effect on glucuronosyl transferase activity. It may be inferred that the ability of isothiocyanates to enhance hepatic microsomal epoxide hydrolase and glucuronosyl transferase activities is dependent on the nature of the side chain. Moreover, in the case of sulforaphane, the naturally occurring R-enantiomer increased both activities, whereas, in contrast, activities were impaired in the case of the S-enantiomer. Finally, intact glucosinolates are potent inducers of epoxide hydrolase and can thus contribute directly to the chemopreventive potential associated with cruciferous vegetable consumption.





Similar content being viewed by others
References
Abdull Razis AF, Iori R, Ioannides C (2010a) The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer. Intern J Cancer (in press)
Abdull Razis AF, Bagatta M, De Nicola GR, Iori R, Ioannides C (2010b) Up-regulation of cytochrome P450 and phase II enzyme systems in rat precision-cut rat lung slices by the intact glucosinolates, glucoraphanin and glucoerucin. Lung Cancer (in press). doi:10.1016/j.lungcan.2010.06.015
Abdull Razis AF, Bagatta M, De Nicola GR, Iori R, Ioannides C (2010c) Intact glucosinolates modulate hepatic cytochrome P450 and phase II conjugation activities and can contribute directly to the chemopreventive activity of cruciferous vegetables. Toxicology 277:74–85
Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG (2004) Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr 134:1134–1138
Bacon JR, Williamson G, Garner RC, Lappin G, Langouët S, Bao Y (2003) Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes. Carcinogenesis 24:1909–1911
Bheemreddy RM, Jeffery EH (2007) The metabolic fate of purified glucoraphanin in F344 rats. J Agric Food Chem 55:2861–2866
Bock KW (2006) UDP-Glucuronosyltransferases. In: Ioannides C (ed) Enzyme systems that metabolise drugs and other xenobiotics. Wiley, Chichester, pp 281–318
Bock KW, White IN (1974) UDP-glucuronyltransferase in perfused rat liver in microsomes: influence of phenobarbital and 3-methylcholanthrene. Eur J Biochem 46:451–459
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Cwik MJ, Wu H, Muzzio M, McCormick DL, Kapetanovic I (2010) Direct quantitation of glucoraphanin in dog and rat plasma by LC-MS/MS. J Pharmaceut Biomed Anal 52:544–549
Dansette PM, DuBois GC, Jerina DM (1979) Continuous fluorometric assay of epoxide hydratase activity. Anal Biochem 97:340–345
Decker M, Arand M, Cronin A (2009) Mammalian epoxide hydrolases in xenobiotic metabolism. Arch Toxicol 83:297–318
Dingley KH, Ubick EA, Chiarappa-Zucca ML, Nowell S, Abel S, Ebeler SE, Mitchell AE, Burns SA, Steinberg FM, Clifford AJ (2003) Effect of dietary constituents with chemopreventive potential on adduct formation of a low dose of the heterocyclic amines PhIP and IQ and phase II enzymes. Nutr Cancer 46:212–221
Getahun SM, Chung FL (1999) Conversion of isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev 8:447–451
Hanlon N, Okpara M, Coldham N, Sauer MJ, Ioannides C (2008a) Modulation of rat hepatic and pulmonary cytochromes P450 and Phase II enzyme systems by erucin, an isothiocyanate structurally related to sulforaphane. J Agric Food Chem 56:7866–7871
Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, Ioannides C (2008b) Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in the rat. Br J Nut 99:559–564
Hanlon N, Poynton CL, Coldham N, Sauer MJ, Ioannides C (2009a) The aliphatic isothiocyanates erucin and sulforaphane do not effectively up-regulate NAD(P)H: quinone oxidoreductase (NQO1) in human liver compared with rat. Molec Nutr Food Res 53:836–844
Hanlon N, Coldham N, Gielbert A, Sauer MJ, Ioannides C (2009b) Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett 284:15–20
Hashemi E, Dobrota M, Till C, Ioannides C (1999) Structural and functional integrity of precision-cut liver slices in xenobiotic metabolism: a comparison of the dynamic organ and multiwell plate culture procedures. Xenobiotica 29:11–25
Hayes JD, Kelleher MO, Eggleston IM (2008) The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 47:73–88
Hecht SS, Carmella SG, Murphy SE (1999) Effects of watercress consumption on urinary metabolites of nicotine smokers. Cancer Epidemiol Biomarkers Prev 8:907–913
Konsue N, Ioannides C (2008) Tissue differences in the modulation of rat cytochromes P450 and phase II conjugation systems by dietary doses of phenethyl isothiocyanate. Food Chem Toxicol 46:3677–3683
Konsue N, Ioannides C (2010) Differential response of four human livers to modulation of phase II enzyme systems by the chemopreventive phytochemical phenethyl isothiocyanate. Molec Nut Food Res 54:426–432
Konsue N, Kirkpatrick J, Kuhnert N, King LJ, Ioannides C (2010) Repeated oral administration modulates the pharmacokinetic behaviour of the chemopreventive agent phenethyl isothiocyanate in rats. Molec Nut Food Res 54:426–432
Navarro SL, Peterson S, Chen C, Makar KW, Schwarz Y, King IB, Li SS, Kestin M, Lampe JW (2009) Cruciferous vegetable feeding alters UGT1A1 activity: diet- and genotype-dependent changes in serum bilirubin in a feeding trial. Cancer Prev Res 2:345–352
Singletary K, MacDonald C (2000) Inhibition of benzo[a]pyrene and 1, 6-dinitropyrene-DNA adduct formation in mammary epithelial cells by dibenzoylmethane and sulforaphane. Cancer Lett 155:47–54
Steinbrecher A, Linseisen L (2009) Dietary intake of individual glucosinolates in participants of the EIPC-Heidelberg cohort study. Ann Nutr Metab 54:87–96
Tang L, Zirpoli GR, Guru K, Moyisch KB, Zhang Y, Ambrosone CB, McCann SE (2008) Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol Biomarkers Prev 17:938–944
Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, De Schrijver R, Hansen M, Gerhäuser C, Mithen R, Dekker M (2009) Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res 53:S219–S265
Visentin M, Tava A, Iori R, Palmieri S (1992) Isolation and Identification of trans-4-(methylthio)-3-butenyl-glucosinolate from radish roots (Raphanus sativus L.). J Agri Food Chem 40:1687–1691
Walters G, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ, Lake BG (2004) Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4, 5-b)pyridine (PhIP) in humans. Carcinogenesis 25:1659–1669
Ye L, Zhang Y (2001) Total intracellular accumulation levels of dietary isothiocyanates determine the activity in elevation of cellular glutathione and phase 2 detoxication enzymes. Carcinogenesis 22:1987–1992
Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C (2005) Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. Int J Cancer 117:356–362
Zhang Y (2004) Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mut Res 555:173–190
Zhang Y, Callaway EC (2002) High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J 364:301–307
Acknowledgments
The authors would like to thank the Malaysian Government for funding this work through a PhD award to one of them (AF Abdull Razis).
Conflict of interest
None of the authors has any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdull Razis, A.F., Bagatta, M., De Nicola, G.R. et al. Induction of epoxide hydrolase and glucuronosyl transferase by isothiocyanates and intact glucosinolates in precision-cut rat liver slices: importance of side-chain substituent and chirality. Arch Toxicol 85, 919–927 (2011). https://doi.org/10.1007/s00204-010-0629-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-010-0629-2