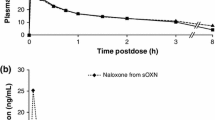
Abstract
Rationale
Prescription opioid abuse has risen dramatically in the United States as clinicians have increased opioid prescribing for alleviation of both acute and chronic pain. Opioid analgesics with decreased risk for abuse are needed.
Objective
Preclinical and clinical studies have shown that opioids combined with ultra-low-dose naltrexone (NTX) may have increased analgesic potency and have suggested reduced abuse or dependence liability. This study addressed whether addition of ultra-low-dose naltrexone might decrease the abuse liability of oxycodone (OXY) in humans.
Materials and methods
This double-blind, placebo-controlled study systematically examined the subjective and physiological effects of combining oral OXY and ultra-low NTX doses in 14 experienced opioid abusers. Seven acute drug conditions given at least 5 days apart were compared in a within-subject crossover design: placebo, OXY 20 mg, OXY 40 mg, plus each of the active OXY doses combined with 0.0001 and 0.001 mg NTX.
Results
The methods were sensitive to detecting opioid effects on abuse liability indices, with significant differences between all OXY conditions and placebo as well as between 20 and 40 mg OXY doses on positive subjective ratings (e.g., “I feel a good drug effect” or “I like the drug”), on observer- and participant-rated opioid agonist effects, and on a drug-versus-money value rating. There were no significant differences or evident trends associated with the addition of either NTX dose on any abuse liability indices.
Conclusions
The addition of ultra-low-dose NTX to OXY did not decrease abuse liability of acutely administered OXY in experienced opioid abusers.


Similar content being viewed by others
References
Alho H, Sinclair D, Vuori E, Holopainen A (2007) Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend 88:75–78
Borsook D, Becerra L, Carlezon WA Jr, Shaw M, Renshaw P, Elman I, Levine J (2007) Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 11:7–20
Buchsbaum MS, Davis GC, Bunney WE Jr (1977) Naloxone alters pain perception and somatosensory evoked potentials in normal subjects. Nature 270:620–622
Chakrabarti S, Regec A, Gintzler AR (2005) Biochemical demonstration of mu-opioid receptor association with Gsα: enhancement following morphine exposure. Brain Res Mol Brain Res 135:217–224
Chindalore VL, Craven RA, Yu KP, Butera PG, Burns LH, Friedmann N (2005) Adding ultra-low-dose naltrexone to oxycodone enhances and prolongs analgesia: a randomized, controlled trial of Oxytrex. J Pain 6:392–399
Cicero TJ, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Senay EC, Woody GE (1999) A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend 57:7–22
Cicero TJ, Inciardi JA, Munoz A (2005) Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain 6:662–672
Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ (2008) Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology 33:1179–1191
Crain SM, Shen KF (1995) Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci 92:10540–10544
Crain SM, Shen KF (2000) Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain 84:121–131
Flugsrud-Breckenridge MR, Gevirtz C, Paul D, Gould HJ 3rd (2007) Medications of abuse in pain management. Curr Opin Anaesthesiol 20:319–324
Gan TJ, Ginsberg B, Glass PS, Fortney J, Jhaveri R, Perno R (1997) Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology 87:1075–81
Griffiths RR, Troisi JR, Silverman K, Mumford GK (1993) Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol 4:3–13
Griffiths RR, Bigelow GE, Ator NA (2003) Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend 70:S41–54
Gueneron JP, Ecoffey C, Carli P, Benhamou D, Gross JB (1988) Effect of naloxone infusion on analgesia and respiratory depression after epidural fentanyl. Anesth Analg 67:35–38
Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD (1987) Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 13:293–308
Largent-Milnes TM, Guo W, Wang H, Burns LH, Vanderah TW (2008) Oxycodone plus ultra-low-dose naltrexone attenuates neuropathic pain and associated mu-opioid receptor-Gs coupling. J Pain 9:700–713
Leri F, Burns LH (2005) Ultra-low-dose naltrexone reduces the rewarding potency of oxycodone and relapse vulnerability in rats. Pharmacol Biochem Behav 82:252–262
Levine JD, Gordon NC, Fields HL (1979) Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature 278:740–741
McCabe SE, Boyd CJ, Young A (2007a) Medical and nonmedical use of prescription drugs among secondary school students. J Adolesc Health 40:76–83
McCabe SE, Morales M, Cranford JA, Delva J, McPherson MD, Boyd CJ (2007b) Race/ethnicity and gender differences in drug use and abuse among college students. J Ethn Subst Abuse 6:75–95
Morley-Forster PK, Clark AJ, Speechley M, Moulin DE (2003) Attitudes toward opioid use for chronic pain: a Canadian physician survey. Pain Res Manag 8:189–194
Nestler EJ (2004) Molecular mechanisms of drug addiction. Neuropharmacology 47(Suppl 1):24–32
Novak S, Nemeth WC, Lawson KA (2004) Trends in medical use and abuse of sustained-release opioid analgesics: a revisit. Pain Med 5:59–65
Olmstead MC, Burns LH (2005) Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology (Berl) 181:576–581
Powell KJ, Abul-Husn NS, Jhamandas A, Olmstead MC, Beninger RJ, Jhamandas K (2002) Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther 300:588–596
Rawal N, Schott U, Dahlstrom B, Inturrisi CE, Tandon B, Sjostrand U, Wennhager M (1986) Influence of naloxone infusion on analgesia and respiratory depression following epidural morphine. Anesthesiology 64:194–201
SAMHSA, Office of Applied Studies (2004) Drug Abuse Warning Network, 2003: Interim National Estimates of Drug-Related Emergency Department Visits. DAWN Series D-26, DHHS Publication No. (SMA) 04-3972
SAMHSA, Office of Applied Studies (2007) Treatment Episode Data Set (TEDS) Highlights - 2007 National Admissions to Substance Abuse Treatment Services. OAS Series S-45, DHHS Publication No. (SMA) 09-4360
Spiller H, Lorenz DJ, Bailey EJ, Dart RC (2009) Epidemiological trends in abuse and misuse of prescription opioids. J Addict Dis 28:130–136
Sproule B, Brands B, Li S, Catz-Biro L (2009) Changing patterns in opioid addiction: characterizing users of oxycodone and other opioids. Can Fam Physician 55:68–69
Stoller KB, Bigelow GE, Walsh SL, Strain EC (2001) Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl) 154:230–242
Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE (1997) The effects of buprenorphine on buprenorphine-maintained volunteers. Psychopharmacology 129:329–338
Strain EC, Stoller K, Walsh SL, Bigelow GE (2000) Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology 148:374–383
Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, Sullivan LE (2008) Gender and non-medical use of prescription opioids: results from a national US survey. Addiction 103:258–268
Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR Jr (2008) The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend 98:191–202
Wang HY, Burns LH (2009) Naloxone’s pentapeptide binding site on filamin A blocks Mu opioid receptor-Gs coupling and CREB activation of acute morphine. PLoS ONE 4:e4282
Wang HY, Friedman E, Olmstead MC, Burns LH (2005) Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gβγ signaling. Neuroscience 135:247–261
Wang HY, Frankfurt M, Burns LH (2008) High-affinity naloxone binding to filamin a prevents mu opioid receptor-Gs coupling underlying opioid tolerance and dependence. PLoS ONE 3:e1554
Webster LR (2007) Oxytrex: an oxycodone and ultra-low-dose naltrexone formulation. Expert Opin Investig Drugs 16:1277–1283
Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N (2006) Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain 7:937–946
Wise RA (1989) Opiate reward: sites and substrates. Neurosci Biobehav Rev 13:129–133
Zacny JP, Gutierrez S (2003) Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology 170:242–54
Acknowledgments
Pain Therapeutics, Inc. provided medications and funding for this study. Company representatives also reviewed the manuscript prior to submission. The authors have no conflicts of interest relevant to this article. Dr. Lanier is an employee of Rock Creek Pharmaceuticals, Inc. and previously worked at Javelin Pharmaceuticals, Inc. In the past 3 years Dr. Bigelow has received consulting payments from Abbott Laboratories, Takeda Pharmaceuticals, Teva Pharmaceuticals, and Acura Pharmaceuticals, and through his university, has received research support from Titan Pharmaceuticals and Pain Therapeutics (for the present study). In the past 3 years, Dr. Strain has received professional services payments from Abbott Laboratories, Center for Substance Abuse Treatment, Friends Research Institute, Grunenthal USA, Progenics Pharmaceuticals, Reckitt Benckiser Pharmaceuticals, Schering Plough, Shire Pharmaceuticals, The Oak Group, and Titan Pharmaceuticals. The experiments conducted here complied with the laws of the United States. The authors have full control of the primary data and agree to allow the journal to review data if requested. We thank Paul Nuzzo for his invaluable help with statistical analysis. As well, we are indebted to and thank the many research assistants and nursing staff who helped conduct the study, manage the data, and prepare manuscript figures, especially Lilian Salinas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pain Therapeutics, Inc. provided medications and funding for this study. Other funding came from NIDA DA07209 and DA023186.
Rights and permissions
About this article
Cite this article
Tompkins, D.A., Lanier, R.K., Harrison, J.A. et al. Human abuse liability assessment of oxycodone combined with ultra-low-dose naltrexone. Psychopharmacology 210, 471–480 (2010). https://doi.org/10.1007/s00213-010-1838-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1838-3