Abstract
Rationale
Recent studies have shown that cannabis may disrupt glutamate (Glu) signaling depressing Glu tone in frequent users. Current evidence have also consistently reported lower Glu-levels in various brain regions, particularly in the medial prefrontal cortex (mPFC) of chronic schizophrenia patients, while findings in early psychosis (EP) are not conclusive. Since cannabis may alter Glu synaptic plasticity and its use is a known risk factor for psychosis, studies focusing on Glu signaling in EP with or without a concomitant cannabis-usage seem crucial.
Objective
We investigate the effect of cannabis use on prefrontal Glu-levels in EP users vs. both EP non-users and healthy controls (HC).
Methods
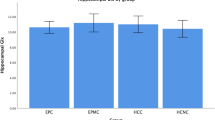
Magnetic resonance spectroscopy was used to measure [GlumPFC] of 35 EP subjects (18 of whom were cannabis users) and 33 HC. For correlative analysis, neuropsychological performances were scored by the MATRICS-consensus cognitive battery.
Results
[GlumPFC] was lower in EP users comparing to both HC and EP non-users (p < 0.001 and p = 0.01, respectively), while no differences were observed between EP non-users and HC. A greater [GlumPFC]-decline with age was observed in EP users (r = −.46; p = 0.04), but not in EP non-users or HC. Among neuropsychological outcomes, working memory was the only domain that differentiates patients depending on their cannabis use, with users having poorer performances.
Conclusions
Cannabis use is associated with reduced prefrontal [GlumPFC] and with a stronger Glu-levels decline with age. Glutamatergic abnormalities might influence the cognitive impairment observed in users and have some relevance for the progression of the disease.




Similar content being viewed by others
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association, Washington, DC
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C (2010) Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 67:255–262
Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE (2002) Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 325:1212–1213
Baumann PS, Crespi S, Marion-Veyron R, Solida A, Thonney J, Favrod J, Bonsack C, Do KQ, Conus P (2013) Treatment and early intervention in psychosis program (TIPP-Lausanne): implementation of an early intervention programme for psychosis in Switzerland. Early Interv Psychiatry 7:322–328
Bernier D, Bartha R, McAllindon D, Hanstock CC, Marchand Y, Dillen KN, Gallant M, Good KP, Tibbo PG (2016) Illness versus substance use effects on the frontal white matter in early phase schizophrenia: a 4Tesla (1)H-MRS study. Schizophr Res 175:4–11
Brandt AS, Unschuld PG, Pradhan S, Lim IA, Churchill G, Harris AD, Hua J, Barker PB, Ross CA, van Zijl PC, Edden RA, Margolis RL (2016) Age-related changes in anterior cingulate cortex glutamate in schizophrenia: a (1)H MRS Study at 7 Tesla. Schizophr Res 172:101–105
Chang L, Cloak C, Yakupov R, Ernst T (2006) Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuro Pharmacol 1:65–76
Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, Pyne-Geithman GJ (2006) N-acetylaspartate as a reservoir for glutamate. Med Hypotheses 67:506–512
Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S (2016) Effect of cannabis on glutamate signalling in the brain: a systematic review of human and animal evidence. Neurosci Biobehav Rev 64:359–381
Deco G, Rolls ET (2003) Attention and working memory: a dynamical model of neuronal activity in the prefrontal cortex. Eur J Neurosci 18:2374–2390
Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Bressan RA, Lacerda AL (2011) Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry 198:442–447
Freeman TP, Morgan CJ, Hindocha C, Schafer G, Das RK, Curran HV (2014) Just say ‘know’: how do cannabinoid concentrations influence users' estimates of cannabis potency and the amount they roll in joints? Addiction 109:1686–1694
Goldman-Rakic PS (1995) Cellular basis of working memory. Neuron 14:477–485
Gruetter R (1993) Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med 29:804–811
Gruetter R, Tkac I (2000) Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 43:319–323
Hanna RC, Shalvoy A, Cullum CM, Ivleva EI, Keshavan M, Pearlson G, Hill SK, Sweeney JA, Tamminga CA, Ghose S (2016) Cognitive function in individuals with psychosis: moderation by adolescent cannabis use. Schizophr Bull 42:1496–1503
Howes O, McCutcheon R, Stone J (2015) Glutamate and dopamine inschizophrenia: an update for the 21st century. J Psychopharmacol 29:97–115
Liemburg E, Sibeijn-Kuiper A, Bais L, Pijnenborg G, Knegtering H, van der Velde J, Opmeer E, de Vos A, Dlabac-De Lange J, Wunderink L, Aleman A (2016) Prefrontal NAA and Glx levels in different stages of psychotic disorders: a 3T 1H-MRS study. Sci Rep 6(1):21873
Lorenzetti V, Solowij N, Yücel M (2015) The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry 79:17–31
Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE (2013) Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull 39:120–129
Mato S, Robbe D, Puente N, Grandes P, Manzoni OJ (2005) Presynaptichomeostatic plasticity rescues long-term depression after chronic Delta9-tetrahydrocannabinol exposure. J Neurosci 25:11619–11627
Mekle R, Mlynarik V, Gambarota G, Hergt M, Krueger G, Gruetter R (2009) MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med 61:1279–1285
Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK (2016) Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry 73:665–674
Mlynarik V, Gambarota G, Frenkel H, Gruetter R (2006) Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med 56:965–970
Muetzel RL, Marjanska M, Collins PF, Becker MP, Valabregue R, Auerbach EJ, Lim KO, Luciana M (2013) In vivo H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage Clin 2:581–589
Mugler JP 3rd, Brookeman JR (1990) Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 15:152–157
Natsubori T, Inoue H, Abe O, Takano Y, Iwashiro N, Aoki Y, Koike S, Yahata N, Katsura M, Gonoi W, Sasaki H, Takao H, Kasai K, Yamasue H (2014) Reduced frontal glutamate + glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull 40:1128–1139
Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S et al (2008) The MATRICS consensus cognitive battery, Part 1: test selection, reliability, and validity. Am J Psychiatry 165:203–213
Pistis M, Porcu G, Melis M, Diana M, Gessa GL (2001) Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur J Neurosci 14:96–102
Preisig M, Fenton BT, Matthey ML, Berney A, Ferrero F (1999) Diagnostic interview for genetic studies (DIGS): inter-rater and test-retest reliability of the French version. Eur Arch Psychiatry Clin Neurosci 249:174–179
Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA (2011) Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. NeuroImage 57:69–75
Prescot AP, Renshaw PF, Yurgelun-Todd DA (2013) Amino butyric acid andglutamate abnormalities in adolescent chronic marijuana smokers. DrugAlcohol Depend 129:232–239
Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P (2008) Elevated 3 T proton MRS glutamate levels associated with poor Continuous Performance Test (CPT-0X) scores and genetic risk for schizophrenia. Schizophr Res 99:218–224
Rigucci S, Marques TR, Di Forti M, Taylor H, Dell'Acqua F, Mondelli V, Bonaccorso S, Simmons A, David AS, Girardi P, Pariante CM, Murray RM, Dazzan P (2016) Effect of high-potency cannabis on corpus callosum microstructure. Psychol Med 46:841–854
Rubino T, Realini N, Braida D, Alberio T, Capurro V, Viganò D, Guidali C, Sala M, Fasano M, Parolaro D (2009) The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res 15:291–302
Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, Sagheddu C, Ligresti A, Tonini R, Di Marzo V, Parolaro D (2015) Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 73:60–69
Tibbo P, Hanstock C, Valiakalayil A, Allen P (2004) 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry 161:1116–1118
Tkac I, Starcuk Z, Choi IY, Gruetter R (1999) In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 41:649–656
Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R (2001) In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med 46:451–456
Treen D, Batlle S, Mollà L, Forcadell E, Chamorro J, Bulbena A, Perez V (2016) Are there glutamate abnormalities in subjects at high risk mental state for psychosis? A review of the evidence. Schizophr 171:166–175
Twamley EW, Palmer BW, Jeste DV, Taylor MJ, Heaton RK (2006) Transient and executive function working memory in schizophrenia. Schizophr Res 87:185–190
Wijtenburg SA, Yang S, Fischer BA, Rowland LM (2015) In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev 51:276–295
Xin L, Mekle R, Fournier M, Baumann PS, Ferrari C, Alameda L, Jenni R, Lu H, Schaller B, Cuenod M, Conus P, Gruetter R, Do KQ (2016) Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophr Bull 42:1185–1196
Yoo SY, Yeon S, Choi C, Kang DH, Lee JM, Shin NY, Jung WH, Choi JS, Jang DP, Kwon JS (2009) Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res 111:86–93
Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J (2005) Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry 39:964–971
Zamberletti E, Beggiato S, Steardo L Jr, Prini P, Antonelli T, Ferraro L, Rubino T, Parolaro D (2014) Alterations of prefrontal cortex GABAergic transmissionin the complex psychotic-like phenotype induced by adolescentdelta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis 63:35–47
Zhang Y, Shen J (2016) Simultaneous quantification of glutamate and glutamine by J-modulated spectroscopy at 3 Tesla. Magn Reson Med 76:725–732
Acknowledgements
We would like to thank all patients and control subjects for their enduring participation. We are grateful for support from the Loterie Romande, Avina Foundation, Damm-Etienne Foundation, Pro Scientia et Arte Foundation, Alamaya Foundation and Sapienza University of Rome—“Avvio alla Ricerca.”
Funding
This work was supported by the Swiss National Science Foundation (320030_122419 to P.C. and K.Q.D), National Center of Competence in Research (NCCR) “SYNAPSY (P.K and L.A - The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (n° 51AU40_125759). P.S.B. was supported by the Leenaards and Jeantet foundation. Magnetic resonance spectroscopy war performed in the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Silvia Rigucci and Lijing Xin are co first authors.
Rights and permissions
About this article
Cite this article
Rigucci, S., Xin, L., Klauser, P. et al. Cannabis use in early psychosis is associated with reduced glutamate levels in the prefrontal cortex. Psychopharmacology 235, 13–22 (2018). https://doi.org/10.1007/s00213-017-4745-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4745-z