Abstract
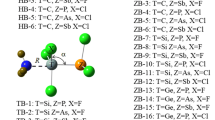
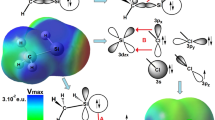
Tetrel-bonded complexes of H n F4−n Si with a N-base for n = 0–4 were explored by MP2 calculations. Configurations with H–Si···N and F–Si···N linear or nearly linear alignment in complexes were considered. Nine sp 3 hybridized nitrogen bases NH3, NH2Cl, NH2F, NHCl2, NCl3, NFCl2, NHF2, NF2Cl, NF3 and nine sp ones NCNH2, NCCH3, NCOH, NP, NCCl, NCH, NCF, NCCN, N2 have been studied. It is shown that binding energies of the complexes depend strongly on the nature of the base involved in the complex. Complexes with NH3 bases present the highest binding energies. In the stronger complexes, the silicon molecules suffer important geometrical distortions. NBO and AIM methodologies have been applied in order to describe properly the intermolecular Si···N contact. F atoms in equatorial position at silicon acid provoke a deviation from linearity of the Si···N electron density bond path trajectory.







Similar content being viewed by others
References
Lehn J-M (2002) Science (Washington, DC, USA) 295(5564):2400
Badjic JD, Nelson A, Cantrill SJ, Turnbull WB, Stoddart JF (2005) Acc Chem Res 38(9):723
Yeagle PL (2014) Biochim Biophys Acta Biomembr 1838(6):1548
Cerny J, Hobza P (2007) Phys Chem Chem Phys 9(39):5291
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Angew Chem Int Ed Engl 34(15):1555
Prins LJ, Reinhoudt DN, Timmerman P (2001) Angew Chem Int Ed 40(13):2382
Steiner T (2002) Angew Chem Int Ed 41(1):48
Grabowski SE (2006) Hydrogen bonding—new insights. Challenges and advances in computational chemistry and physics, vol 3. Springer Netherlands, Amsterdam
Singh SK, Das A (2015) Phys Chem Chem Phys 17(15):9596
Schreiner PR, Chernish LV, Gunchenko PA, Tikhonchuk EY, Hausmann H, Serafin M, Schlecht S, Dahl JEP, Carlson RMK, Fokin AA (2011) Nature (London, UK) 477(7364):308
Murray JS, Lane P, Politzer P (2009) J Mol Model 15(6):723
Murray JS, Riley KE, Politzer P, Clark T (2010) Aust J Chem 63(12):1598
Politzer P, Murray JS, Concha MC (2008) J Mol Model 14(8):659
Politzer P, Murray JS, Clark T (2013) PCCP 15(27):11178
Azofra LM, Scheiner S (2015) J Chem Phys 142(3):034307
Bauzá A, Mooibroek TJ, Frontera A (2013) Angew Chem Int Ed 52(47):12317
Grabowski SJ (2014) PCCP 16(5):1824
Del Bene JE, Alkorta I, Elguero J (2015) The pnicogen bond in review: structures, binding energies, bonding properties, and spin–spin coupling constants of complexes stabilized by pnicogen bonds. In: Scheiner S (ed) Noncovalent forces. Challenges and advances in computational chemistry and physics, vol 19. Springer, Berlin. doi:10.1007/978-3-319-14163-3_8
Scheiner S (2013) Acc Chem Res 46(2):280
Esrafili MD, Mohammadian-Sabet F (2015) Chem Phys Lett 628:71
Esrafili MD, Mohammadian-Sabet F (2015) J Mol Model 21(3):1
Esrafili MD, Mohammadian-Sabet F (2016) Chem Phys Lett 645:32
Metrangolo P, Resnati G (2015) Halogen bonding I. Impact on materials chemistry and life sciences. Topics in current chemistry, vol 358. Springer, Berlin
Politzer P, Lane P, Concha MC, Ma Y, Murray JS (2007) J Mol Model 13(2):305
Alkorta I, Rozas I, Elguero J (2001) J Phys Chem A 105(4):743
Ruoff RS, Emilsson T, Jaman AI, Germann TC, Gutowsky HS (1992) J Chem Phys 96(5):3441
Urban RD, Rouillé G, Takami M (1997) J Mol Struct 413:511
Alkorta I, Elguero J, Fruchier A, Macquarrie DJ, Virgili A (2001) J Organomet Chem 625(2):148
Rossi AR, Jasinski JM (1990) Chem Phys Lett 169(5):399
Yamamura M, Kano N, Kawashima T, Matsumoto T, Harada J, Ogawa K (2008) J Org Chem 73(21):8244
Hagemann M, Berger RJF, Hayes SA, Stammler H-G, Mitzel NW (2008) Chem A Eur J 14(35):11027
Vojinović K, McLachlan LJ, Hinchley SL, Rankin DWH, Mitzel NW (2004) Chem A Eur J 10(12):3033
Marín-Luna M, Alkorta I, Elguero J (2015) J Organomet Chem 794:206
Korlyukov AA, Lyssenko KA, Antipin MY, Kirin VN, Chernyshev EA, Knyazev SP (2002) Inorg Chem 41(20):5043
Marin-Luna M, Alkorta I, Elguero J (2016) J Phys Chem A 120(4):648
Esrafili MD, Mohammadirad N, Solimannejad M (2015) Chem Phys Lett 628:16
Del Bene JE, Alkorta I, Elguero J (2015) J Phys Chem A 119(22):5853
Bene JED, Alkorta I, Elguero J (2015) J Phys Chem A 119(12):3125
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian IWC. Gaussian-09, Revision A.01
Møller C, Plesset MS (1934) Phys Rev 46(7):618
Papajak E, Zheng J, Xu X, Leverentz HR, Truhlar DG (2011) J Chem Theory Comput 7(10):3027
Kendall RA, Dunning TH, Harrison RJ (1992) J Chem Phys 96(9):6796
Lu T, Chen F (2012) J Comput Chem 33(5):580
Jmol (2013) An open-source java viewer for chemical structures in 3D vhwjoaS
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Popelier PL (2000) Atoms in molecules: an introduction. Prentice Hall, London
Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules: from solid state to DNA and drug design. WILEY-VCH, Weinham
AIMAll (Version 14.11.23) TAK, TK Gristmill Software, Overland Park KS, USA, 2014 (aim.tkgristmill.com)
Glendening ED, Landis CR, Weinhold F (2013) NBO 6.0: natural bond orbital analysis program. J Comput Chem 34(16):1429–1437
Murray JS, Concha MC, Politzer P (2011) J Mol Model 17(9):2151
Politzer P, Murray JS, Clark T (2015) J Mol Model 21(3):52
Knop O, Boyd RJ, Choi SC (1988) J Am Chem Soc 110(22):7299
Gibbs GV, Hill FC, Boisen MB, Downs RT (1998) Phys Chem Miner 25(8):585
Espinosa E, Alkorta I, Elguero J, Molins E (2002) J Chem Phys 117(12):5529
Alkorta I, Barrios L, Rozas I, Elguero J (2000) THEOCHEM 496(1–3):131
Knop O, Rankin KN, Boyd RJ (2001) J Phys Chem A 105(26):6552
Mata I, Molins E, Alkorta I, Espinosa E (2007) J Phys Chem A 111(28):6425
Mata I, Alkorta I, Molins E, Espinosa E (2010) Chem A Eur J 16(8):2442
Rozas I, Alkorta I, Elguero J (2000) J Am Chem Soc 122(45):11154
Acknowledgements
This work was carried out with financial support from the Ministerio de Economía y Competitividad (Project No. CTQ2015-63997-C2-2-P) and Comunidad Autónoma de Madrid (Project FOTOCARBON, ref S2013/MIT-2841). Computer, storage and other resources from the CTI (CSIC) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published as part of the special collection of articles derived from the 10th Congress on Electronic Structure: Principles and Applications (ESPA-2016).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marín-Luna, M., Alkorta, I. & Elguero, J. A theoretical study of the H n F4−n Si:N-base (n = 1–4) tetrel-bonded complexes. Theor Chem Acc 136, 41 (2017). https://doi.org/10.1007/s00214-017-2069-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-017-2069-z