Abstract
A three-phase LPME (liquid-phase microextraction) method for the enantioselective analysis of venlafaxine (VF) metabolites (O-desmethylvenlafaxine (ODV) and N-desmethylvenlafaxine (NDV) in microsomal preparations is described for the first time. The assay involves the chiral HPLC separation of drug and metabolites using a Chiralpak AD column under normal-phase mode of elution and detection at 230 nm. The LPME procedure was optimized using multifactorial experiments and the following optimal condition was established: sample agitation at 1,750 rpm, 20 min of extraction, acetic acid 0.1 mol/L as acceptor phase, 1-octanol as organic phase and donor phase pH adjustment to 10.0. Under these conditions, the mean recoveries were 41% and 42% for (−)-(R)-ODV and (+)-(S)-ODV, respectively, and 47% and 48% for (−)-(R)-NDV and (+)-(S)-NDV, respectively. The method presented quantification limits of 200 ng/mL and it was linear over the concentration range of 200–5,000 ng/mL for all analytes. The validated method was employed to study the in vitro biotransformation of VF using rat liver microsomal fraction. The results demonstrated the enantioselective biotransformation of VF.

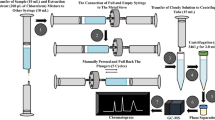
Chromatogram showing the chiral metabolites N-desmethylvenlafaxine (peaks 3 and 4) and O-desmethylvenlafaxine (peaks 5 and 6) formed from the biotransformation of venlafaxine (peaks 1 and 2) in rat liver microsomes




Similar content being viewed by others
References
Liu W, Cai H, Li H (2007) J Chromatogr B 850:405–411
Fanali S, Rudaz S, Veuthey JL, Desiderio C (2001) J Chromatogr A 919:195–203
Ciusani E, Zullino DF, Eap CB, Brawand-Amey M, Brocard M, Baumann P (2004) J Psychopharmacol 18:550–566
Wang CP, Howell SR, Scatina J, Sisenwinw SF (1992) Chirality 4:84–90
Hermann M, Hendset M, Fosaas K, Hjerpset M, Refsum H (2008) Eur J Clin Pharmacol 64:483–487
Kamath J, Handratta V (2008) Expert Rev Neurother 8:1787–1797
Gex-Fabry M, Rudaz S, Balant-Gorgia AE, Brachet A, Veuthey JL, Balant LP, Bertschy G (2002) Eur J Clin Pharmacol 58:323–331
Eap CB, Lessard E, Baumann P, Brawand-Amey M, Yessine MA, O´Hara G, Turgeon J (2003) Pharmacogenetics 13:39–47
Wen L, Feng W, Huan-de L (2007) J Chromatogr B 850:183–189
Rudaz S, Stella C, Balant-Gorgia AE, Fanali S, Veuthey JL (2000) J Pharm Bio Anal 23:107–115
Ho TS, Pedersen-Bjergaard S, Rasmussen KE (2006) J Chromatogr Sci 44:308–316
Da Fonseca P, Freitas LAP, Pinto LFR, Pestana CR, Bonato PS (2008) J Chromatogr B 875:161–167
Pedersen-Bjergaard S, Rasmussen KE (1999) Anal Chem 71:2650–2656
Psillakis E, Kalogerakis N (2003) Trends Anal Chem 22:565–574
Rasmussen KE, Pedersen-Bjergaard S (2004) Trends Anal Chem 23:1–10
De Oliveira ARM, Magalhães IRS, De Santana FJM, Bonato PS (2008) Quim Nova 31:637–644
Lake BG (1987) In: Snell K, Mullock B (eds.) Biochemical toxicology—a practical approach, Oxford, Washington
Zhao L, Zhu L, Lee HK (2002) J Chromatogr A 963:239–248
Pedersen-Bjergaard S, Rasmussen KE (2005) J Chromatogr B 817:3–12
Andersen S, Halvorsen TG, Pedersen-Bjergaard S, Rasmussen KE (2002) J Chromatogr A 963:303–312
FDA—Guidance for industry: bioanalytical methods validation (May 2001)
Brandon EFA, Raap CD, Meijerman I, Beijnen JH, Schellens JHM (2003) Toxicol Appl Pharmacol 189:233–246
Tingle MD, Helsby NA (2006) Environ Toxicol Pharmacol 21:184–190
Riley RJ, Grime K (2004) Drug Discov Today 1:365–372
Acknowledgments
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for granting financial support and research fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Fonseca, P., Bonato, P.S. Chiral HPLC analysis of venlafaxine metabolites in rat liver microsomal preparations after LPME extraction and application to an in vitro biotransformation study. Anal Bioanal Chem 396, 817–824 (2010). https://doi.org/10.1007/s00216-009-3271-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3271-1