Abstract
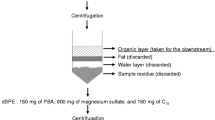
The application of the quick easy cheap effective rugged and safe (QuEChERS) method has generally been used in the preparation of produce samples (e.g., lettuce and oranges) for pesticide analysis. In the present study, the QuEChERS method was successfully applied to the determination of the natural pyrethrins (cinerin I and II, jasmolin I and II, and pyrethrin I and II), as well as two pyrethroid insecticides, cypermethrin and deltamethrin, in fin and non-fin fish products. Analysis of these compounds was performed using gas chromatography–mass spectrometry. Although cypermethrin is not and has not been registered for pest control use in aquaculture operations in Canada, cypermethrin was detected in domestically produced salmon. Cypermethrin was detected in seven of the 18 Canadian farmed salmon samples (39%), although it was not detected in any wild domestic salmon (n = 3). Cypermethrin concentrations ranged from 0.3 to 6.5 ng/g in the positive samples. It was not, however, observed in any imported fish product or any other domestically produced fish product.

20 ng/g standard prepared in salmon matrix


Similar content being viewed by others
References
Anadón A, Martínez-Larrañaga MR, Martínez MA (2009) Use and abuse of pyrethrins and synthetic pyrethroids in veterinary medicine. Vet J 182:7–20
Vázquez PP, Mughari AR, Galera MM (2008) Solid-phase microextraction (SPME) for the determination of pyrethroids in cucumber and watermelon using liquid chromatography combined with post-column photochemically induced fluorimetry derivatization and fluorescence detection. Anal Chim Acta 607:74–82
Barbini DA, Vanni F, Girolimetti S, Dommarco R (2007) Development of an analytical method for the determination of the residues of four pyrethroids in meat by GC-ECD and confirmation GC-MS. Anal Bioanal Chem 389:1791–1798
Yáñez L, Ortiz-Pérez D, Batres LE, Borja-Aburto VH, Díaz-Barriga F (2002) Levels of dichlorodiphenyltrichloroethane and deltamethrin in humans and environmental samples in malarious areas of Mexico. Environ Res A 88:174–181
López-López T, Gil-Garcia MD, Martínez-Vidal JL, Martínez-Galera M (2001) Determination of pyrethroids in vegetables by HPLC using continuous on-line post-elution photoirradiation with fluorescence detection. Analytica Chimica Acta 447:101–111
Berg A-GT, Horsberg TE (2009) Plasma concentrations of emamectin benzoate after Slice treatments of Atlantic salmon (Salmo salar): differences between fish, cages, sites and seasons. Aquaculture 288:22–26
Giorgi M, Cugini I, Meucci V, Bestini S, Giuliani M, Soldani G (2005) New HPLC and GC-MS methods for the investigation of cypermethrin in edible portions of fish: development, validation and comparison. Vet Res Commun 29(Suppl 2):293–295
Gowland B, Webster L, Fryer R, Davies I, Moffat C, Stagg R (2002) Uptake and effects of the cypermethrin-containing sea lice treatment Excis in the marine mussel, Mytilus edulis. Environ Pollut 120:805–811
Sorensen LK, Hansen H (2003) Determination of cypermethrin in salmon by LC-APCI-MS. Anal Lett 36:191–201
Hart JL, Thacker JRM, Braidwood JC, Fraser NR, Matthews JE (1997) Novel cypermethrin formulation for the control of the sea lice on salmon (Salmo salar). Vet Rec 140:179–181
State of Maine (2009) Information sheet on use of therapeutic agents in marine finfish aquaculture. State of Maine Department of Marine Resources. Aquaculture Environmental Monitoring Program. February, 19, 2009. http://www.mar.dfo-mpo.gc.ca/science/review/1996/Page/Page%20e.html. Accessed 31 October, 2009
Page, F, Chang B, Ernst W, Julien G, Losier R (1996) Research on the dispersion of sea louse pesticides in the marine environment. http://www.mar.dfo-mpo.gc.ca/science/review/1996/Page/Page%20e.html. Accessed 2 November 2009
Santerre CR, Ingram R, Lewis GW, Davies JT, Lane LG, Grodner RM, Wei C-I, Bush PB, Xu DH, Shelton J, Alley EG, Hinshaw JM (2000) Organochlorines, organophosphates, and pyrethroids in channel catfish, rainbow trout, and red swamp crayfish from aquaculture facilities. J Food Sci 65:231–235
You J, Lydy MJ (2004) Simultaneous determination of pyrethroid, organophosphate, and organochlorine pesticides in fish tissue using tandem solid-phase extraction clean up. Intern J Environ Anal Chem 84:559–571
Tao C-J, Hu J-Y, Li J-Z, Zheng S-S, Liu W, Li C-J (2009) Multi-residue determination of pesticides in vegetables by gas chromatography/ion trap mass spectrometry. Bull Environ Contam Toxicol 82:111–115
Vonderheide AP, Kauffman PE, Heiber TE, Brisbin JA, Melnyk LJ, Morgan JN (2009) Development of an analytical scheme for the determination of pyrethroid pesticides in composite diet samples. J Agric Food Chem 57:2096–2104
Woudneh MB, Ou Z, Sekela M, Tuominen T, Gledhill M (2009) Pesticide multiresidues in waters of the Lower Fraser Valley, British Columbia, Canada. Part 1. Surface Water. J Environ Qual 38:948–954
Mekebri A, Crane DB, Blondina GJ, Oros DR, Rocca JL (2008) Extraction and analysis methods for the determination of pyrethroid insecticides in surface water, sediments and biological tissues at environmentally relevant concentrations. Bull Environ Contam Toxicol 80:455–460
Al-Makkawy HK, Madbouly MD (1999) Persistence and accumulation of some organic insecticides in Nile water and fish. Resour Conserv Recycling 27:105–115
Ostrea EM Jr, Bielawski DM, Posecion NC Jr, Corrion M, Villanueva-Uy E, Bernardo RC, Jin Y, Janisse JJ, Ager JW (2009) Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environ Res 109:116–122
Stahnke H, Reemtsma T, Alder L (2009) Compensation of matrix effects by postcolumn infusion of a monitor substance in multiresidue analysis with LC-MS/MS. Anal Chem 81:2185–2192
US FDA (US Food and Drug Administration) (2006) Total Diet Study Market Baskets 1991–3 through 2003–4. http://www.fda.gov/downloads/Food/FoodSafety/FoodContaminantsAdulteration/TotalDietStudy/UCM184303.pdf. Accessed 19 October 2009
Payá P, Anastassiades M, Mack D, Sigalova I, Tasdelen B, Oliva J, Barba A (2007) Analysis of pesticide residues using quick easy cheap effective rugged and safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and mass spectrometric detection. Anal Bioanal Chem 389:1697–1714
Lehotay SJ, de Kok A, Hemstra M, Bodegraven P (2005) Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas chromatography and liquid chromatography and mass spectrometric detection. J AOAC Intern 88:595–614
Lehotay SJ, Maštovská K, Lightfield AR (2005) Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J AOAC Intern 88:615–629
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Intern 86:412–431
Lehotay SJ, Maštovská K, Yun SJ (2005) Evaluation of two fast and easy methods for pesticide residue analysis in fatty food matrixes. J AOAC Intern 88:630–638
Lesueur C, Knittl P, Gartner M, Mentler A, Fuerhacker M (2008) Analysis of 140 pesticides from conventional farming foodstuff samples after extraction with the modified QuEChERS method. Food Control 19:906–914
Rawn DFK, Krakalovich T, Forsyth DS, Roscoe V (2009) Analysis of fin and non-fin fish products for azamethiphos and dichlorvos residues from the Canadian retail market. Intern J Food Sci Technol 44:1510–1516
Rawn DFK, Forsyth DS, Ryan JJ, Breakell K, Verigin V, Nicolidakis H, Hayward S, Laffey P, Conacher HBS (2006) PCB, PCDD and PCDF residues in fin and non-fin fish products from the Canadian retail market 2002. Sci Tot Environ 359:101–110
Tittlemier SA, Forsyth D, Breakall K, Verigin V, Ryan JJ, Hayward S (2004) Polybrominated diphenyl ethers in retail fish and seafood samples purchased from Canadian markets. J Agric Food Chem 52:7740–7745
Kruve A, Herodes K, Leito I (2010) Optimization of electrospray interface and quadupole ion trap mass spectrometer parameters in pesticide liquid chromatography/electrospray mass spectrometry analysis. Rapid Commun Mass Spectrom 24:919–926
Georgakopoulos P, Foteinopoulou E, Athanasopoulos P, Drosinos E, Skandamis P (2007) Recoveries of four representative organophosphorus pesticides from 18 plant products belonging to different botantical categories: implications for matrix effects. Food Addit Contam 24:360–368
Niessen WMA, Manini P, Andreoli R (2006) Matrix effects in quantitative pesticide analysis using liquid chromatography–mass spectrometry. Mass Spectrom Rev 25:881–899
Frenich AG, Vidal JLM, Moreno JLF, Romero-Gonzalez R (2009) Compensation for matrix effects in gas chromatography–tandem mass spectrometry using a single point standard addition. J Chromatogr A 1216:4798–4808
Cunha SC, Lehotay SJ, Maštovská K, Fernandes JO, Beatriz M, Oliveira PP (2007) Evaluation of the QuEChERS sample preparation approach for the analysis of pesticide residues in olives. J Sep Sci 30:620–632
Poole CF (2007) Matrix-induced response enhancement in pesticide residue analysis by gas chromatography. J Chromatogr A 1158:241–250
Maštovská K, Lehotay SJ, Anastassiades M (2005) Combination of analyte protectants to overcome matrix effects in routine GC analysis of pesticide residues in food matrixes. Anal Chem 77:8129–8137
Government of Canada (2009) Health Canada’s list of MRLs regulated under the Pest Control Product Act. http://www.hc-sc.gc.ca/cps-spc/pest/protect-proteger/food-nourriture/mrl-lmr-eng.php. Accessed 14 October 2009
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rawn, D.F.K., Judge, J. & Roscoe, V. Application of the QuEChERS method for the analysis of pyrethrins and pyrethroids in fish tissues. Anal Bioanal Chem 397, 2525–2531 (2010). https://doi.org/10.1007/s00216-010-3786-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3786-5