Abstract
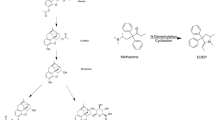
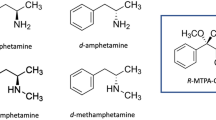
In order to develop an analytical method for the discrimination of dextromethorphan (an antitussive medicine) from its enantiomer, levomethorphan (a narcotic) in biological samples, chiral analyses of these drugs and their O-demethyl and/or N-demethyl metabolites in rat plasma, urine, and hair were carried out using LC-MS/MS. After the i.p. administration of dextromethorphan or levomethorphan to pigmented hairy male DA rats (5 mg/kg/day, 10 days), the parent compounds and their three metabolites in plasma, urine and hair were determined using LC-MS/MS. Complete chiral separation was achieved in 12 min on a Chiral CD-Ph column in 0.1% formic acid–acetonitrile by a linear gradient program. Most of the metabolites were detected as being the corresponding O-demethyl and N, O-didemethyl metabolites in the rat plasma and urine after the hydrolysis of O-glucuronides, although obvious differences in the amounts of these metabolites were found between the dextro and levo forms. No racemation was observed through O- and/or N-demethylation. In the rat hair samples collected 4 weeks after the first administration, those differences were more clearly detected and the concentrations of the parent compounds, their O-demethyl, N-demethyl, and N, O-didemethyl metabolites were 63.4, 2.7, 25.1, and 0.7 ng/mg for the dextro forms and 24.5, 24.6, 2.6, and 0.5 ng/mg for the levo forms, respectively. In order to fully investigate the differences of their metabolic properties between dextromethorphan and levomethorphan, DA rat and human liver microsomes were studied. The results suggested that there might be an enantioselective metabolism of levomethorphan, especially with regard to the O-demethylation, not only in DA rat but human liver microsomes as well. The proposed chiral analyses might be applied to human samples and could be useful for discriminating dextromethorphan use from levomethorphan use in the field of forensic toxicology, although further studies should be carried out using authentic human samples.






Similar content being viewed by others
References
Mutschler J, Koopmann A, Grosshans M, Hermann D, Mann K, Kiefer F (2010) Dtsch Arztebl Int 107:537–540
Chyka PA, Erdman AR, Manoguerra AS, Christianson G, Booze LL, Nelson LS, Woolf AD, Cobaugh DJ, Caravati EM, Scharman EJ, Troutman WG (2007) Clin Toxicol 45:662–677
Banken JA, Foster H (2008) Ann NY Acad Sci 1139:402–411
Shin EJ, Lee PH, Kim HJ, Nabeshima T, Kim HC (2008) J Pharmacol Sci 106:22–27
Bryner JK, Wang UK, Hui JW, Bedodo M, MacDougall C, Anderson IB (2006) Arch Pediatr Adolesc Med 160:1217–1222
Miller SC (2005) Addict Biol 10:325–327
Logan BK, Goldfogel G, Hamilton R, Kuhlman J (2009) J Anal Toxicol 33:99–103
Rammer L, Holmgren P, Sandler H (1988) Forensic Sci Int 37:233–236
Woods JW, Carney J (1978) NIDA Res Monogr 18:54–66
Trescot AM, Datta S, Lee M, Hansen H (2008) Pain Physician 11:S133–S153
Jacqz-Aigrain E, Funck-Brentano C, Cresteil T (1993) Pharmacogenetics 3:197–204
Schmid B, Bircher J, Preisig R, Küpfer A (1985) Clin Pharmacol Ther 38:618–624
Gorski JC, Jones DR, Wrighton SA, Hall SD (1994) Biochem Pharmacol 48:173–182
Köppel C, Tenczer J, Arndt I, Ibe K (1987) Arzneimittelforschung 37:1304–1306
Lutz U, Bittner N, Lutz RW, Lutz WK (2008) J Chromatogr B 871:349–356
Kristensen HT (1998) J Pharm Biomed Anal 18:827–838
Aumatell A, Wells RJ (1993) J Chromatogr Sci 31:502–508
Lin SY, Chen CH, Ho HO, Chen HH, Sheu MT (2007) J Chromatogr B 859:141–146
Bendriss EK, Markoglou N, Wainer IW (2001) J Chromatogr B 754:209–215
Afshar M, Rouini MR, Amini M (2004) J Chromatogr B 802:317–322
Hendrickson HP, Gurley BJ, Wessinger WD (2003) J Chromatogr B. B 788:261–268
Kim SC, Chung H, Lee SK, Park YH, Yoo YC, Yun YP (2006) Forensic Sci Int 161:185–188
Spanakis M, Vizirianakis IS, Mironidou-Tzouveleki M, Niopas I (2009) Biomed Chromatogr 23:1131–1137
Rodrigues WC, Wang G, Moore C, Agrawal A, Vincent MJ, Soares JR (2008) J Anal Toxicol 32:220–226
Kim EM, Lee JS, Park MJ, Choi SK, Lim MA, Chung HS (2006) Forensic Sci Int 161:198–201
Bagheri H, Es-haghi A, Rouini MR (2005) J Chromatogr B 818:147–157
Eichhold TH, McCauley-Myers DL, Khambe DA, Thompson GA, Hoke SH 2nd (2007) J Pharm Biomed Anal 43:586–600
Kuhlenbeck DL, Eichold TH, Hoke SH 2nd, Baker TR, Mensen R, Wehmeyer KR (2005) Eur J Mass Spectrom 11:199–208
Lutz U, Völkel W, Lutz RW, Lutz WK (2004) J Chromatogr B 813:217–225
Vengurlekar SS, Heitkamp J, McCush F, Velagaleti PR, Brisson JH, Bramer SL (2002) J Pharm Biomed Anal 30:113–124
Sunouchi M, Fukuhara M, Ohno Y, Takanaka A (1988) J Toxicol Sci 13:193–204
Ozawa S, Ohta K, Miyajima A, Kurebayashi H, Sunouchi M, Shimizu M, Murayama N, Matsumoto Y, Fukuoka M, Ohno Y (2000) Xenobiotica 10:1005–1017
Kikura-Hanajiri R, Kawamura M, Saisho K, Kodama Y, Goda Y (2007) J Chromatogr B 855:121–126
Kikura-Hanajiri R, Kawamura M, Miyajima A, Sunouchi M, Goda Y (2010) Forensic Sci Int 198:62–69
Bochner F, Somogyi AA, Chen ZR (1994) Xenobiotica 24:543–552
Kerry NL, Somogyi AA, Mikus G, Bochner F (1993) Biochem Pharmacol 45:833–839
Zysset T, Zeugin T, Küpfer A (1988) Biochem Pharmacol 37:3155–3160
Nakahara Y, Takahashi K, Kikura R (1995) Biol Pharm Bull 18:1223–1227
Nakahara Y, Ochiai T, Kikura R (1992) Arch Toxicol 66:446–449
Acknowledgments
Part of this work was supported by a Health and Labor Sciences Research Grant from the Ministry of Health, Labor and Welfare in Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Forensic Toxicology with Guest Editors Frank T. Peters, Hans H. Maurer, and Frank Musshoff.
Rights and permissions
About this article
Cite this article
Kikura-Hanajiri, R., Kawamura, M., Miyajima, A. et al. Chiral analyses of dextromethorphan/levomethorphan and their metabolites in rat and human samples using LC-MS/MS. Anal Bioanal Chem 400, 165–174 (2011). https://doi.org/10.1007/s00216-011-4707-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-4707-y