Abstract
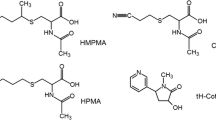
Acrylonitrile (AN), a widely used industrial chemical also found in tobacco smoke, has been classified as a possible human carcinogen (group 2B) by the International Agency for Research on Cancer. AN can be detoxified by glutathione S-transferase (GST) to form glutathione (GSH) conjugates in vivo. It can be metabolically activated by cytochrome P450 2E1 to form 2-cyanoethylene oxide, which can also be detoxified by GST to generate GSH conjugates. The GSH conjugates can be further metabolized to mercapturic acids (MAs), namely, N-acetyl-S-(2-cyanoethyl)cysteine (CEMA), N-acetyl-S-(2-hydroxyethyl)cysteine (HEMA), and N-acetyl-S-(1-cyano-2-hydroxyethyl)cysteine (CHEMA). This study developed an ultraperformance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) method to quantitatively profile the major AN urinary metabolites (CEMA, HEMA, and CHEMA) to assess AN exposure, as well as analyze urinary cotinine (COT) as an indicator for tobacco smoke exposure. The limits of quantitation were 0.1, 0.1, 1.0, and 0.05 μg/L for HEMA, CEMA, CHEMA, and COT, respectively. This method was applied to analyze the three AN-derived MAs in 36 volunteers with no prior occupational AN exposure. Data analysis showed significant correlations between the level of COT and the levels of these MAs, suggesting them as biomarkers for exposure to low levels of AN. The results demonstrate that a highly specific and sensitive UPLC-MS/MS method has been successfully developed to quantitatively profile the major urinary metabolites of AN in humans to assess low AN exposure.

Simultaneous quantitation of 3 acrylonitrile-derived mercapturic acids and cotinine in human urine samples with on-line solid-phase extraction LC-MS/MS.



Similar content being viewed by others
References
IARC (1999) Summary of data reported and evaluation. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide, vol 71. IARC, Lyon, France
Hoffmann D, Hoffmann I, El-Bayoumy K (2001) Chem Res Toxicol 14:767–790
Léonard A, Gerber GB, Stecca C, Rueff J, Borba H, Farmer PB, Sram RJ, Czeizel AE, Kalina I (1999) Mutat Res 436:263–283
National Research Council (1995) Biologic markers in urinary toxicology. National Academies Press, Washington
Sumner SC, Selvaraj L, Nauhaus SK, Fennell TR (1997) Chem Res Toxicol 10:1152–1160
Jakubowski M, Linhart I, Pielas G, Kopecký J (1987) Br J Ind Med 44:834–840
Vermeulen NP, de Jong J, van Bergen EJ, van Welie RT (1989) Arch Toxicol 63:173–184
Calafat AM, Barr DB, Pirkle JL, Ashley DL (1999) J Expo Anal Environ Epidemiol 9:336–342
Linhart I, Smejkal J, Novák J (1988) Arch Toxicol 61:484–488
Schettgen T, Musiol A, Alt A, Ochsmann E, Kraus T (2009) Anal Bioanal Chem 393:969–981
De Rooij BM, Commandeur JNM, Vermeulen NPE (1998) Biomarkers 3:239–303
Barr DB, AshleyDL J (1998) Anal Toxicol 22:96–104
Haufroid V, Merz B, Hofmann A, Tschopp A, Lison D, Hotz P (2007) Cancer Epidemiol Biomarkers Prev 16:796–802
Schettgen T, Musiol A, Kraus T (2008) Rapid Commun Mass Spectrom 22:2629–2638
Ding YS, Blount BG, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, Ashley DL (2009) Chem Res Toxicol 22:1018–1025
Schulte PA, Waters M (1999) Ann N Y Acad Sci 895:101–111
Benowitz NL (1996) Epidemiol Rev 18:188–204
Jaffe M (1886) Hoppe-Seylers Z Physiol Chem 10:391
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75:3019–3030
Matuszewski BK, Constanzer ML, Chavez-Eng CM (1998) Anal Chem 70:882–889
Food and Drug Administration (2001) Guidance for industry bioanalytical method validation. CVM & CDER, FDA, US DOH
Chung CC (2004) Analytical method validation and instrument performance verification. John Wiley & Sons, Inc., Publication, Hoboken
Kataoka H, Inoue R, Yagi K, Saito K (2009) J Pharm Biomed Anal 49:108–114
Fan Z, Xie F, Xia Q, Wang S, Ding L, Liu H (2008) Chromatographia 68:623–627
Chadwick CA, Keevil B (2007) Ann Clin Biochem 44:455–462
Hoofnagle AN, Laha TJ, Rainey PM, Sadrzadeh SM (2007) Am J Clin Pathol 126:880–887
Heavner DL, Richardson JD, Morgan WT, Ogden MW (2005) Biomed Chromatogr 19:312–328
Xu X, Iba MM, Weisel PC (2004) Clin Chem 50:2323–2330
Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, Hurt RD (2002) Clin Chem 48:1460–1471
Tuomi T, Johnsson T, Reijula K (1999) Clin Chem 452:2164–2172
Minet E, Cheung F, Errington G, Sterz K, Scherer G (2011) Biomarkers 16:89–96
Eckert E, Schmid K, Schaller B, Hiddemann-Koca K, Drexler H, Goen T (2011) Int J Hyg Environ Health 214:196–204
Acknowledgements
The authors gratefully appreciate the technical support and assistance with the UPLC-MS/MS analyses provided by Dr. Wei-Chung Shih. This study was supported in part by grants from the Institute of Occupational Safety and Health, Council of Labor Affairs (grant no. IOSH98-A313), the National Sciences Council (no. NSC98-2314-B-002-082-MY3), and the Environmental and Occupational Health Center at National Taiwan University, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chia-Fang Wu and Shi-Nian Uang are co-first authors
Rights and permissions
About this article
Cite this article
Wu, CF., Uang, SN., Chiang, SY. et al. Simultaneous quantitation of urinary cotinine and acrylonitrile-derived mercapturic acids with ultraperformance liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 402, 2113–2120 (2012). https://doi.org/10.1007/s00216-011-5661-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5661-4