Abstract
The fluorescent tag 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC; AccQ Fluor reagent kit from Waters) is a commercial N-terminal label for proteinogenic amino acids (AAs), designed for reversed-phase separation and quantification of the AA racemates. The applicability of AQC-tagged AAs and AA-type zwitterionic compounds was tested for enantiomer separation on the tert-butyl carbamate modified quinine and quinidine based chiral stationary phases, QN-AX and QD-AX employing polar-organic elution conditions. The investigated test analytes included the enantiomers of the positional isomers of isoleucine (Ile), threonine, homoserine, and 4-hydroxyproline. Furthermore, β-AAs, cyclic, and heterocyclic AAs including trans-2-amino-cyclohexane carboxylic acid and trans-2-aminocyclohexyl sulfonic acid, phenylalanine derivatives substituted with halides with increasing electronegativity and 3,4-dihydroxyphenylalanine, cysteine-related derivatives including homocysteic acid, methionine sulfone, cysteine-S-acetic acid, and cysteine-S-acetamide as well as a small range of aminophosphonic acids were enantioseparated. A mechanistic interaction study of AQC-AAs in comparison with fluoresceine isothiocyanate-labeled AAs was performed. The chiral and chemoselective recognition processes involved in enantiomer separation and retention was systematically discussed. Special emphasis was set on the influential factors exhibited by the chemistry, branching position, and spatial properties of the investigated zwitterionic analytes. The general interest to separate and distinguish between different types of branched-chained AAs and metabolic side products thereof lies in the toxicity of some of these compounds, which makes for instance allo–Ile an attractive candidate in disease-related biomarker research.

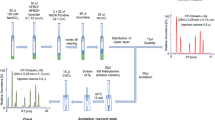
Separation of the four AQC-tagged isomers of 4-hydroxyproline (trans-D, cis-D, trans-L and cis-L) on the chiral stationary phase QD-AX







Similar content being viewed by others
References
Kaiser K, Benner R (2005) Hydrolysis-induced racemization of amino acids. Limnol Oceanogr: Methods 3:318–325
Hamase K, Morikawa A, Ohgusu T, Lindner W, Zaitsu K (2007) Comprehensive analysis of branched aliphatic d-amino acids in mammals using an integrated multi-loop two-dimensional column-switching high-performance liquid chromatographic system combining reversed-phase and enantioselective columns. J Chromatogr A 1143(1–2):105–111
Han H, Miyoshi Y, Ueno K, Okamura C, Tojo Y, Mita M, Lindner W, Zaitsu K, Hamase K (2011) Simultaneous determination of d-aspartic acid and d-glutamic acid in rat tissues and physiological fluids using a multi-loop two-dimensional HPLC procedure. J Chromatogr B 879(29):3196–3202
Armstrong DW, Gasper M, Lee SH, Zukowski J, Ercal N (1993) d-amino acid levels in human physiological fluids. Chirality 5(5):375–378
Hamase K (2011) Analysis and biological relevance of d-amino acids and relating compounds. J Chromatogr B 879(29):3077
Bernal JL, Nozal MJ, Toribio L, Diego JC, Ruiz A (2005) A comparative study of several HPLC methods for determining free amino acid profiles in honey. J Sep Sci 28(9–10):1039–1047
Gogami Y, Okada K, Oikawa T (2011) High-performance liquid chromatography analysis of naturally occurring d-amino acids in sake. J Chromatogr B 879(29):3259–3267
Friedman M (2010) Origin, microbiology, nutrition, and pharmacology of d-amino acids. Chem Biodiv 7(6):1491–1530
Vranova V, Zahradnickova H, Janous D, Skene KR, Matharu AS, Rejsek K, Formanek P (2012) The significance of d-amino acids in soil, fate and utilization by microbes and plants: review and identification of knowledge gaps. Plant Soil 354(1–2):21–39
Hamase K, Morikawa A, Zaitsu K (2002) d-amino acids in mammals and their diagnostic value. J Chromatogr B 781(1–2):73–91
Mustafa Asif K, Kim Paul M, Snyder Solomon H (2004) d-serine as a putative glial neurotransmitter. Neuron Glia Biol 1(3):275–281
Reischl RJ, Hartmanova L, Carrozzo M, Huszar M, Fruehauf P, Lindner W (2011) Chemoselective and enantioselective analysis of proteinogenic amino acids utilizing N-derivatization and 1-D enantioselective anion-exchange chromatography in combination with tandem mass spectrometric detection. J Chromatogr, A 1218(46):8379–8387
Schadewaldt P, Bodner-Leidecker A, Hammen H-W, Wendel U (1999) Significance of l-alloisoleucine in plasma for diagnosis of maple syrup urine disease. Clin Chem 45(10):1734–1740
Tojo Y, Hamase K, Nakata M, Morikawa A, Mita M, Ashida Y, Lindner W, Zaitsu K (2008) Automated and simultaneous two-dimensional micro-high-performance liquid chromatographic determination of proline and hydroxyproline enantiomers in mammals. J Chromatogr B 875(1):174–179
Jeong J-S, Sim H-J, Lee Y-M, Yoon H-R, Kwon H-J, Hong S-P (2011) Chromatographic diagnosis of maple syrup urine disease by measuring the l-alloisoleucine/l-phenylalanine ratio in dried blood spots. J Chromatogr B 879(22):2171–2174
Kaufman DS, Miller GH (1995) Isoleucine epimerization and amino acid composition in molecular-weight separations of Pleistocene Genyornis eggshell. Geochim Cosmochim Acta 59(13):2757–2765
Katane M, Homma H (2011) d-aspartate—an important bioactive substance in mammals: a review from an analytical and biological point of view. J Chromatogr B 879(29):3108–3121
Richter K, Egger R, Kreil G (1987) d-alanine in the frog skin peptide dermorphin is derived from l-alanine in the precursor. Science 238(4824):200–202
Kirschner DL, Green TK (2009) Separation and sensitive detection of d-amino acids in biological matrices. J Sep Sci 32(13):2305–2318
Schurig V (2002) Chiral separations using gas chromatography. Trends Anal Chem 21(9–10):647
Waldhier MC, Dettmer K, Gruber MA, Oefner PJ (2010) Comparison of derivatization and chromatographic methods for GC-MS analysis of amino acid enantiomers in physiological samples. J Chromatogr B 878(15–16):1103–111222
Algermissen B, Neundel M, Riedel E (1989) Determination of amino acids by fluorescence HPLC. GIT Fachz Lab 33(9):783–786, 789–790
Brueckner H, Wittner R, Godel H (1991) Fully automated high-performance liquid chromatographic separation of dl-amino acids derivatized with o-phthaldialdehyde together with N-isobutyryl-cysteine. Application to food samples. Chromatographia 32(7–8):383–388
Bhushan R, Bruckner H (2011) Use of Marfey's reagent and analogs for chiral amino acid analysis: assessment and applications to natural products and biological systems. J Chromatogr B 879(29):3148–3161
Cohen SA, Michaud DP (1993) Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem 211(2):279–287
Cohen SA (2005) Quantitation of amino acids as 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate derivatives. J Chromatogr Libr 70:242–267
van Wandelen C, Cohen SA (1997) Using quaternary high-performance liquid chromatography eluent systems for separating 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate-derivatized amino acid mixtures. J Chromatogr, A 763(1–2):11–22
He Y-Y, Zhao L-J, Yuan H-Y, Xu Z-M, Tang Y, Xiao D, Choi MMF (2011) HPLC with in-capillary optical fiber laser-induced fluorescence detection of picomolar amounts of amino acids by precolumn fluorescence derivatization with fluorescein isothiocyanate. Chromatographia 74(7–8):541–547
Van den Beld CMB, Lingeman H, Van Ringen GJ, Tjaden UR, Van der Greef J (1988) Laser-induced fluorescence detection in liquid chromatography after preliminary derivatization of carboxylic acid and primary amino groups. Anal Chim Acta 205(1–2):15–27
Ueno K, Hamase K, Miyoshi Y, Mita M, Kadota Y, Nishio Y, Zaitsu K (2009) RP-HPLC separation of NBD-derivatives of hydrophilic amino acids using a semi-micro monolithic-ODS column. Chromatography 30(suppl 1):9–10
Miyoshi Y, Hamase K, Tojo Y, Mita M, Konno R, Zaitsu K (2009) Determination of d-serine and d-alanine in the tissues and physiological fluids of mice with various d-amino-acid oxidase activities using two-dimensional high-performance liquid chromatography with fluorescence detection. J Chromatogr B 877(24):2506–2512
Liu JH (1994) Determination of amino acids by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate and high-performance liquid chromatography with ultra violet detection. J Chromatogr A 670:59–64
Soriano BD, Tam L-TT LHS, Valladares VG (2012) A fluorescent-based HPLC assay for quantification of cysteine and cysteamine adducts in Escherichia coli-derived proteins. J Chromatogr B 880:27–33
Bosch L, Alegria A, Farre R (2006) Application of the 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) reagent to the RP-HPLC determination of amino acids in infant foods. J Chromatogr B 831(1–2):176–183
Lammerhofer M, Lindner W (2008) Liquid chromatographic enantiomer separation and chiral recognition by cinchona alkaloid-derived enantioselective separation materials. Adv Chromatogr 46:1–107
Laemmerhofer M, Lindner W (1996) Quinine and quinidine derivatives as chiral selectors. I. Brush type chiral stationary phases for high-performance liquid chromatography based on cinchonan carbamates and their application as chiral anion exchangers. J Chromatogr, A 741(1):33–48
Mandl A, Nicoletti L, Lammerhofer M, Lindner W (1999) Quinine versus carbamoylated quinine-based chiral anion exchangers. A comparison regarding enantioselectivity for N-protected amino acids and other chiral acids. J Chromatogr, A 858(1):1–11
Maier NM, Nicoletti L, Laemmerhofer M, Lindner W (1999) Enantioselective anion exchangers based on cinchona alkaloid-derived carbamates: influence of C8/C9 stereochemistry on chiral recognition. Chirality 11(7):522–528
Chen S, Pawlowska M, Armstrong DW (1994) HPLC enantioseparation of di- and tripeptides on cyclodextrin bonded stationary phases after derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC). J Liq Chromatogr 17(3):483–497
Pawlowska M, Chen S, Armstrong DW (1993) Enantiomeric separation of fluorescent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, tagged amino acids. J Chromatogr 641(2):257–265
Cladrowa-Runge S, Rizzi A (1997) Enantioseparation of 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate-derivatized-amino acids by capillary zone electrophoresis using native and substituted Î2-cyclodextrins as chiral additives. I. Discussion of optimum separation conditions. J Chromatogr A 759(1 + 2):157–165
Zarbl E, Lammerhofer M, Hammerschmidt F, Wuggenig F, Hanbauer M, Maier NM, Sajovic L, Lindner W (2000) Direct liquid chromatographic enantioseparation of chiral a- and b-aminophosphonic acids employing quinine-derived chiral anion exchangers: determination of enantiomeric excess and verification of absolute configuration. Anal Chim Acta 404(2):169–177
Cohen SA (2001) Amino acid analysis using precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. Methods Mol Biol 159:39–47
Peter A, Vekes E, Arki A, Tourwe D, Lindner W (2003) Direct high-performance liquid chromatographic enantioseparation of α-substituted proline analogues on a quinine-derived chiral anion-exchanger stationary phase. J Sep Sci 26(12–13):1125–1132
Reischl RJ, Lindner W (2012) Methoxyquinoline labeling—a new strategy for the enantioseparation of all chiral proteinogenic amino acids in 1-dimensional liquid chromatography using fluorescence and tandem mass spectrometric detection. J Chromatogr A 1269:262–269
Horak J, Maier NM, Lindner W (2004) Investigations on the chromatographic behavior of hybrid reversed-phase materials containing electron donor-acceptor systems II. Contribution of π-π aromatic interactions. J Chromatogr A 1045(1–2):43–58
Ihara H, Sagawa T, Nakashima K-I, Mitsuishi K, Goto Y, Chowdhury J, Sakaki S (2000) Enhancement of diastereomer selectivity using highly-oriented polymer stationary phase. Chem Lett 2:128–129
Horak J, Lindner W (2004) Investigations on the chromatographic behavior of hybride reversed phase materials containing electron donor-acceptor systems. I. Contrbution of sulfur-aromatic interactions. J Chromatogr, A 1043:177–194
Horak J, Lindner W (2008) Contribution of sulfonyl-aromatic and sulfonic acid-aromatic interactions in novel sulfonyl/sulfonic acid-embedded reversed phase materials. J Chromatogr A 1191(1–2):141–156
Pirkle WH, Pochapsky TC (1989) Considerations of chiral recognition relevant to the liquid chromatography separation of enantiomers. Chem Rev 89(2):347–362
Zahradnickova H, Jegorov A, Trnka T, Zelenka K (2008) Thiosugars—derivatization agents for chiral resolution of homoleucines. J Sep Sci 31(1):133–136
Shoulders MD, Kotch FW, Choudhary A, Guzei IA, Raines RT (2010) The aberrance of the 4S diastereomer of 4-hydroxyproline. J Am Chem Soc 132(31):10857–10865
Abe I, Nakahara T (1996) Enantiomer separation of amino acids as their N-alkyloxycarbonyl alkylamide derivatives by chiral phase capillary GC. J High Resolut Chromatogr 19(9):511–514
Simek P, Husek P, Zahradnickova H (2012) Heptafluorobutyl chloroformate-based sample preparation protocol for chiral and nonchiral amino acid analysis by gas chromatography. Methods Mol Biol 828:137–152, Amino Acid Analysis
Kaufman DS, Manley WF (1998) A new procedure for determining dl amino acid ratios in fossils using reversed phase liquid chromatography. Quat Geochem 17:987–1000
Hamase K, Miyoshi Y, Ueno K, Han H, Hirano J, Morikawa A, Mita M, Kaneko T, Lindner W, Zaitsu K (2010) Simultaneous determination of hydrophilic amino acid enantiomers in mammalian tissues and physiological fluids applying a fully automated micro-two-dimensional high-performance liquid chromatographic concept. J Chromatogr A 1217(7):1056–1062
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Amino Acid Analysis with guest editor Toshimasa Toyo'oka.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 723 kb)
Rights and permissions
About this article
Cite this article
Hellinger, R., Horak, J. & Lindner, W. Enantioseparation of 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate tagged amino acids and other zwitterionic compounds on cinchona-based chiral stationary phases. Anal Bioanal Chem 405, 8105–8120 (2013). https://doi.org/10.1007/s00216-013-7121-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7121-9