Abstract
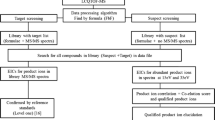
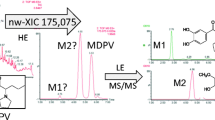
The development of a liquid chromatography high-resolution mass spectrometry quadrupole-time-of-flight (LC-HRMS-QTOF) method for the analysis of new stimulant designer drugs (e.g. phenethylamine, amphetamine, cathinone and piperazine derivatives) and common drugs of abuse (e.g. ketamine and ritalinic acid) in urine is reported. Sample preparation was carried out by a fast and convenient salting-out liquid-liquid extraction (SALLE) procedure. The data was generated by a preferred target list combined with untargeted data-dependent acquisition recording additional sample information (i.e. not listed metabolites of target compounds or not database-stored drugs). The identification is realised by a fully automated data extraction algorithm, taking into account accurate mass spectra, fragment masses and retention times. Method validation comprised selectivity, linearity, accuracy, stability, determination of the limit of detection (LOD) and limit of quantification (LOQ) and evaluation of matrix effects and recoveries for a total set of 39 compounds. Acceptable quantitative results were obtained for 35 of the 39 analytes. Exemplarily, application of the additional untargeted data-dependent acquisition mode enabled the identification of metabolites of the preferred target list compounds ketamine and methylenedioxypyrovalerone (MDPV) without use of reference standards. Therefore, improvement of the database is feasible with every positive library hit. The approach presented here provides a very useful tool for the combined targeted and untargeted analysis of drugs of abuse in biological matrices such as urine.





Similar content being viewed by others

References
Zuba D (2012) Identification of cathinones and other active components of ‘legal highs’ by mass spectrometric methods. Trends Anal Chem 32:15–30
Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V (2010) Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom 45(10):1186–1194. doi:10.1002/jms.1811
Katagi M, Kamata T, Zaitsu K, Shima N, Kamata H, Nakanishi K, Nishioka H, Miki A, Tsuchihashi H (2010) Metabolism and toxicologic analysis of tryptamine-derived drugs of abuse. Ther Drug Monit 32(3):328–331. doi:10.1097/FTD.0b013e3181dcb40c
Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168(2):458–470. doi:10.1111/j.1476-5381.2012.02145.x
Shulgin A (1991) PiHKAL—a chemical love story. Transform Press, Berkeley, California
Shulgin A (1997) TiHKAL—the continuation. Transform Press, Berkeley, California
Zaitsu K, Katagi M, Kamata H, Nakanishi K, Shima N, Kamata T, Nishioka H, Miki A, Tatsuno M, Tsuchihashi H (2010) Simultaneous analysis of six novel hallucinogenic (tetrahydobenzodifuranyl)aminoalkanes (FLYs) and (benzodifuranyl)aminoalkanes (DragonFLYs) by GC-MS, LC-MS and LC-MS-MS. Forensic Toxicol 28:9–18
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) Identification of two new-type designer drugs, piperazine derivative MT-45 (I-C6) and synthetic peptide Noopept (GVS-111), with synthetic cannabinoid A-834735, cathinone derivative 4-methoxy-alpha-PVP, and phenethylamine derivative 4-methylbuphedrine from illegal products. Forensic Toxicol online first: 3rd June 2013
Uchiyama N, Shimokawa Y, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2013) Two new synthetic cannabinoids, AM 2201 benzimidazole analog (FUBIMINA) and (4-methylpiperazin-1-yl)(1-pentyl-1H-indol-3-yl)methanone (MEPIRAPIM), and three phenethylamine derivative, 25H-NBOMe 3,4,5-trimethoxybenzyl analog, 25B-NBOMe, and C-N-NBOMe, identified in illegal products. Forensic Toxicol online first: 5th November 2013
Dresen S, Ferreiros N, Gnann H, Zimmermann R, Weinmann W (2010) Detection and identification of 700 drugs by multi-target screening with a 3200 Q TRAP LC-MS/MS system and library searching. Anal Bioanal Chem 396(7):2425–2434. doi:10.1007/s00216-010-3485-2
Dresen S, Gergov M, Politi L, Halter C, Weinmann W (2009) ESI-MS/MS library of 1,253 compounds for application in forensic and clinical toxicology. Anal Bioanal Chem 395(8):2521–2526. doi:10.1007/s00216-009-3084-2
Maurer HH, Pfleger K, Weber A (2011) Mass spectral and GC data of drugs, poisons, pesticides, pollutants and their metabolites, vol 4th Edition edn. Wiley-VCH, Weinheim, Germany
Roesner P, Junge T, Westphal F, Fritschi G (2013) Mass spectra of designer drugs 2014. Wiley-VCH, Weinheim, Germany
Concheiro M, Anizan S, Ellefsen K, Huestis MA (2013) Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography-high resolution mass spectrometry. Anal Bioanal Chem 405(29):9437–9448. doi:10.1007/s00216-013-7386-z
Broecker S, Herre S, Wust B, Zweigenbaum J, Pragst F (2011) Development and practical application of a library of CID accurate mass spectra of more than 2,500 toxic compounds for systematic toxicological analysis by LC-QTOF-MS with data-dependent acquisition. Anal Bioanal Chem 400(1):101–117. doi:10.1007/s00216-010-4450-9
Sundstrom M, Pelander A, Angerer V, Hutter M, Kneisel S, Ojanpera I (2013) A high-sensitivity ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method for screening synthetic cannabinoids and other drugs of abuse in urine. Anal Bioanal Chem 405(26):8463–8474. doi:10.1007/s00216-013-7272-8
Yanes EG, Lovett DP (2012) High-throughput bioanalytical method for analysis of synthetic cannabinoid metabolites in urine using salting-out sample preparation and LC-MS/MS. J Chromatogr B: Analyt Technol Biomed Life Sci 909:42–50. doi:10.1016/j.jchromb.2012.10.013
Peters FT (2007) Stability of analytes in biosamples—an important issue in clinical and forensic toxicology? Anal Bioanal Chem 388(7):1505–1519. doi:10.1007/s00216-007-1267-2
Tsujikawa K, Mikuma T, Kuwayama K, Miyaguchi H, Kanamori T, Iwata YT, Inoue H (2012) Degradation pathways of 4-methylmethcathinone in alkaline solution and stability of methcathinone analogs in various pH solutions. Forensic Sci Int 220(1–3):103–110. doi:10.1016/j.forsciint.2012.02.005
Sorensen LK (2011) Determination of cathinones and related ephedrines in forensic whole-blood samples by liquid-chromatography-electrospray tandem mass spectrometry. J Chromatogr B: Analyt Technol Biomed Life Sci 879(11–12):727–736. doi:10.1016/j.jchromb.2011.02.010
Johnson RD, Botch-Jones SR (2013) The stability of four designer drugs: MDPV, mephedrone, BZP and TFMPP in three biological matrices under various storage conditions. J Anal Toxicol 37(2):51–55. doi:10.1093/jat/bks138
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Bioanalytical method validation—a revisit with a decade of progress. Pharm Res 17(12):1551–1557
Peters FT, Hartung M, Herbold M, Schmitt G, Daldrup T, Musshoff F (2009) Anhang B zur Richtlinie der GTFCh zur Qualitätssicherung bei forensisch-toxikologischen Untersuchungen Anforderungen an die Validierung von Analysenmethoden. ToxichemKrimtech 76(3):185–208
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75(13):3019–3030
Baselt RC (2011) Disposition of toxic drugs and chemicals in man, 9th edn. Biomedical Publications, Seal Beach, California
Wang KC, Shih TS, Cheng SG (2005) Use of SPE and LC/TIS/MS/MS for rapid detection and quantitation of ketamine and its metabolite, norketamine, in urine. Forensic Sci Int 147(1):81–88. doi:10.1016/j.forsciint.2004.03.031
Bijlsma L, Sancho JV, Hernandez F, Niessen WM (2011) Fragmentation pathways of drugs of abuse and their metabolites based on QTOF MS/MS and MS(E) accurate-mass spectra. J Mass Spectrom 46(9):865–875. doi:10.1002/jms.1963
Niessen WM (2011) Fragmentation of toxicologically relevant drugs in positive-ion liquid chromatography-tandem mass spectrometry. Mass Spectrom Rev 30(4):626–663. doi:10.1002/mas.20332
Meyer MR, Du P, Schuster F, Maurer HH (2010) Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. J Mass Spectrom 45(12):1426–1442. doi:10.1002/jms.1859
Strano-Rossi S, Cadwallader AB, de la Torre X, Botre F (2010) Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MDPV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 24(18):2706–2714. doi:10.1002/rcm.4692
Acknowledgments
The authors gratefully acknowledge Mr. Sebastian Bröcker for his technical assistance and support. Michael Paul thanks Mrs. Heike Franke (Rudolf-Böhm-Institut für Pharmakologie, Leipzig) and Mrs. Adelgunde Gräfe (Institut für Rechtsmedizin, Leipzig) from the University of Leipzig, Germany, for accepting this work as final thesis in the postgraduate programme in Toxicology and Environmental Protection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, M., Ippisch, J., Herrmann, C. et al. Analysis of new designer drugs and common drugs of abuse in urine by a combined targeted and untargeted LC-HR-QTOFMS approach. Anal Bioanal Chem 406, 4425–4441 (2014). https://doi.org/10.1007/s00216-014-7825-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7825-5