Abstract
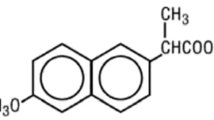
The new sample preparation concept “Parallel artificial liquid membrane extraction (PALME)” was evaluated for extraction of the acidic drugs ketoprofen, fenoprofen, diclofenac, flurbiprofen, ibuprofen, and gemfibrozil from human plasma samples. Plasma samples (250 μL) were loaded into individual wells in a 96-well donor plate and diluted with HCl to protonate the acidic drugs. The acidic drugs were extracted as protonated species from the individual plasma samples, through corresponding artificial liquid membranes each comprising 2 μL of dihexyl ether, and into corresponding acceptor solutions each comprising 50 μL of 25 mM ammonia solution (pH 10). The liquid membranes and the acceptor solutions were located in a 96-well filter plate, which was sandwiched with the 96-well donor plate during extraction. Parallel extraction of several samples was performed for 15 to 60 min, followed by high-performance liquid chromatography-ultraviolet detection of the individual acceptor solutions. Important PALME parameters including the chemical composition of the liquid membrane, extraction time, and sample pH were optimized, and the extraction performance was evaluated. Except for flurbiprofen, exhaustive extraction was accomplished from plasma. Linearity was obtained for all six drugs in the range 0.025–10 μg/mL, with r 2 values ranging between 0.998 and 1.000. Precision data were in the range 3–22 % RSD, and accuracy data were within 72–130 % with spiked plasma samples. Based on the current experiences, PALME showed substantial potential for future high-throughput bioanalysis of non-polar acidic drugs.





Similar content being viewed by others
References
Kataoka H, Saito K, Yokoyama A (2010) In: Pawliszyn J, Lord HL (eds) Handbook of sample preparation. Wiley, Hoboken, pp 285–312
Arthur CL, Pawliszyn J (1990) Anal Chem 62:2145–2148
Jeannot MA, Cantwell FC (1997) Anal Chem 69:235–239
Pedersen-Bjergaard S, Rasmussen KE (1999) Anal Chem 71:2650–2656
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghae E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116:1–6
Spietelun A, Marcinkowski L, de la Guardia M, Namiesnik J (2014) Talanta 119:34–45
Bello-Lopez MA, Ramos-Payan M, Ocana-Gonzalez JA, Fernandez-Torres R, Callejon-Mochon M (2012) Anal Lett 45:804–830
Ghambarian M, Yamini Y, Esrafili A (2012) Microchim Acta 177:271–294
Nuhu AA, Basheer C, Saad B (2011) J Chromatogr B 879:1180–1188
Zhao L, Lee HK, Majors RE (2010) LCGC N Am 28:580–591
Gjelstad A, Rasmussen KE, Parmer MP, Pedersen-Bjergaard S (2013) Bioanalysis 5:1377–1385
Pedersen-Bjergaard S, Rasmussen KE (2000) Electrophoresis 21:579–585
Ramos Payán M, Bello López MA, Fernández-Torres R, Pérez Bernal JL, Callejón Mochón M (2009) Anal Chim Acta 653:184–190
Larsson N, Petersson E, Rylander M, Jönsson JÅ (2009) Anal Methods 1:59–67
Villar Navarro M, Ramos Payán M, Fernández-Torres R, Bello López MA, Callejón Mochón M, Guiráum Pérez A (2011) Electrophoresis 32:2107–2113
Abolfazl S, Larsson E, Yamini Y, Jönsson JÅ (2011) J Chromatogr A 1218:1331–1339
Villar Navarro M, Ramos Payán M, Fernández-Torres R, Bello López MA (2013) Biomed Chromatogr 27:246–253
Schulz M, Schmoldt A (2003) Pharmazie 58:447–474
Gjelstad A, Taherkhani H, Rasmussen KE, Pedersen-Bjergaard S, Majors RE (2011) LCGC N Am 29:1038–1045
US Department of Health and Human Services, US FDA, Center for Drug Evaluation and Research, Center for Veterinary Medicine (2001) Guidance for industry, bioanalytical method validation. Rockville, MD, USA
de Juan A, Fonrodona G, Casassas E (1997) Trends Anal Chem 16:52–62
Acknowledgments
The authors want to thank the Research Council of Norway for the support of parts of this project (NFR 234396). M. Roldán-Pijuán wishes to thank the Ministry for the award of a FPU Research Training Fellowship (Grant AP2009-3499), and gratefully acknowledges funding of her stay in Oslo at the School of Pharmacy to conduct the research reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roldán-Pijuán, M., Pedersen-Bjergaard, S. & Gjelstad, A. Parallel artificial liquid membrane extraction of acidic drugs from human plasma. Anal Bioanal Chem 407, 2811–2819 (2015). https://doi.org/10.1007/s00216-015-8505-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8505-9