Abstract
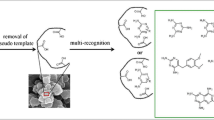
Three fragmental templates, including 2,4-diamino-6-methyl-1,3,5-triazine (DMT), cyromazine (CYR), and trimethoprim (TME), were used to prepare the fragment molecularly imprinted polymers (FMIPs), respectively, in polar ternary porogen which was composed of ionic liquid ([BMIM]BF4), methanol, and water. The morphology, specific surface areas, and selectivity of the obtained FMIPs for fragmental analogues were systematically characterized. The experimental results showed that the FMIPs possessed the best specific recognition ability to the relative template and the greatest imprinting factor (IF) was 5.25, 6.69, and 7.11 of DMT on DMT-MIPs, CYR on CYR-MIPs, and TME on TME-MIPs, respectively. In addition, DMT-MIPs also showed excellent recognition capability for fragmental analogues including CYR, melamine (MEL), triamterene (TAT), and TME, and the IFs were 2.08, 3.89, 2.18, and 2.60, respectively. The effects of pH and temperature on the retention of the fragmental and structural analogues were studied in detail. Van’t Hoff analysis indicated that the retention and selectivity on FMIPs were an entropy-driven process, i.e., steric interaction. The resulting DMT-MIPs were used as a solid-phase extraction material to enrich CYR, MEL, TAT, and TME in different bio-matrix samples for high-performance liquid chromatography analysis. The developed method had acceptable recoveries (86.8–98.6 %, n = 3) and precision (2.7–4.6 %) at three spiked levels (0.05–0.5 μg g−1).






Similar content being viewed by others
References
Chen LX, Xu SF, Li JH (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40(5):2922–2942
Toth B, Horvai G (2012) Chromatography, solid-phase extraction, and capillary electrochromatography with MIPs. Top Curr Chem 325:267–306
Kulsing C, Knob R, Macka M, Junor P, Boysen RI, Hearn MTW (2014) Molecular imprinted polymeric porous layers in open tubular capillaries for chiral separations. J Chromatogr A 1354(8):85–91
Orozco J, Cortes A, Cheng G, Sattayasamisathit S, Gao W, Feng XM, Shen YF, Wang J (2013) Molecularly imprinted polymer-based catalytic micromotors for selective protein transport. J Am Chem Soc 135(14):5536–5539
Li B, Xu JJ, Hall AJ, Haupt K, Bui BTS (2014) Water-compatible silica sol–gel molecularly imprinted polymer as a potential delivery system for the controlled release of salicylic acid. J Mol Recognit 27(9):559–565
Fuchs Y, Soppera O, Mayes AG, Haupt K (2013) Holographic molecularly imprinted polymers for label-free chemical sensing. Adv Mater 25(4):566–570
Chen FF, Xie XY, Shi YP (2013) Preparation of magnetic molecularly imprinted polymer for selective recognition of resveratrol in wine. J Chromatogr A 1300(26):112–118
Kawaguchi M, Hayatsu Y, Nakata H, Ishii Y, Ito R, Saito K, Nakazawa H (2005) Molecularly imprinted solid phase extraction using stable isotope labeled compounds as template and liquid chromatography-mass spectrometry for trace analysis of bisphenol A in water sample. Anal Chim Acta 539(1–2):83–89
Bognar J, Szucs J, Dorko Z, Horvath V, Gyurcsanyi RE (2013) Nanosphere lithography as a versatile method to generate surface-imprinted polymer films for selective protein recognition. Adv Funct Mater 23(37):4703–4709
Cacho C, Turiel E, Martin-Esteban A, Ayala D, Perez-Conde C (2006) Semi-covalent imprinted polymer using propazine methacrylate as template molecule for the clean-up of triazines in soil and vegetable samples. J Chromatogr A 1114(2):255–262
Berffren C, Bayoudh S, Sherrington D, Ensing K (2000) Use of molecularly imprinted solid-phase extraction for the selective clean-up of clenbuterol from calf urine. J Chromatogr A 889(1–2):105–110
Ellwanger A, Berggren C, Bayoudh S, Crecenzi C, Karlsson L, Owens PK, Ensing K, Cormack P, Sherrington D, Sellergren B (2001) Evaluation of methods aimed at complete removal of template from molecularly imprinted polymers. Analyst 126(6):784–792
Kubo T, Kuroda K, Tominaga Y, Naito T, Sueyoshi K, Hosoya K, Otsuka K (2014) Effective determination of a pharmaceutical, sulpiride, in river water by online SPE-LC-MS using a molecularly imprinted polymer as a preconcentration medium. J Pharm Biomed 89(15):111–117
Cleand D, McCluskey A (2013) The use of effective fragment potentials in the design and synthesis of molecularly imprinted polymers for the group recognition of PCBs. Org Biomol Chem 11(28):4646–4656
Yan HY, Liu ST, Gao MM, Sun N (2013) Ionic liquids modified dummy molecularly imprinted microspheres as solid phase extraction materials for determination of clenbuterol and clorprenaline in urine. J Chromatogr A 1294(14):10–16
Zhao XY, Zhang HW, Liang ZJ, Shu YP, Liang Y (2013) Selective recognition of triamterene in biological samples by molecularly imprinted monolithic column with a pseudo template employed. J Sep Sci 36(9–10):1501–1508
Zaidi SA (2013) Dual-templates molecularly imprinted monolithic for the evaluation of serotonin and histamine in CEC. Electrophresis 34(9–10):1375–1382
Jing T, Wang Y, Dai Q, Xia H, Niu JW, Hao QL, Mei SR, Zhou YK (2010) Preparation of mixed-templates molecularly imprinted polymers and investigation of the recognition ability for tetracycline antibiotics. Biosens Bioelectron 25(10):2218–2224
Prasad BB, Jauhari D (2015) A dual-template biomimetic molecularly imprinted dendrimer-based piezoelectric sensor for ultratrace analysis of organochlorine pesticides. Sensor Actuators B Chem 207(Part A):542–551
Wang XH, Fang QX, Liu SP, Chen L (2012) Preparation of a magnetic molecularly imprinted polymer with pseudo template for rapid simultaneous determination of cyromazine and melamine in bio-matrix samples. Anal Bioanal Chem 404(5):1555–1564
Nezhadali A, Mojarrab M (2015) Fabrication of an electrochemical molecularly imprinted polymer triamterene sensor based on multivariate optimization using multi-walled carbon nanotubes. J Electroanal Chem 744(1):85–94
Zhang HW, Guo JW, Li K, Wang WS, Wu YP, Lu QW, Zhai HY (2012) On-line determination of trimethoprim in human serum by molecularly imprinted monolithic column with melamine employed as analogue template. Chin J Anal Chem 40(5):699–704
Quaglis M, Chenon K, Hall AJ, Lorenti ED, Sellergren B (2001) Target analogue imprinted polymers with affinity for folic acid and related compounds. J Am Chem Soc 123(10):2146–2154
He LM, Su YJ, Zheng YQ, Huang XH, Wu L, Liu YH, Zeng ZL, Chen ZL (2009) Novel cyromazine imprinted polymer applied to the solid-phase extraction of melamine from feed and milk samples. J Chromatogr A 1216(34):6196–6203
Song XL, Li JH, Wang JT, Chen LX (2009) Quercetin molecularly imprinted polymers: preparation, recognition characteristics and properties as sorbent for solid-phase extraction. Talanta 80(2):694–702
Pakade V, Lindahl S, Chimuka L, Turner C (2012) Molecularly imprinted polymers targeting quercetin in high-temperature aqueous solutions. J Chromatogr A 1230(23):15–23
Ban L, Han X, Wang XH, Huang YP, Liu ZS (2013) Carprofen-imprinted monolith prepared by reversible addition-fragmentation chain transfer polymerization in room temperature ionic liquid. Anal Bioanal Chem 405(26):8597–8605
Holland N, Frisby J, Owens E, Hughes H, Duggan P, McLoughlin P (2010) The influence of polymer morphology on the performance of molecularly imprinted polymers. Polymer 51(7):1578–1584
Dauwe C, Sellergren B (1996) Influence of template basicity and hydrophobicity on the molecularly recognition properties of molecularly imprinted polymers. J Chromatogr A 753(2):191–200
Gavazzini A, Marchetti N, Guzzinati R, Pasti L, Ciogli A, Gasprrini F, Lagana A (2014) Understanding mixed-mode retention mechanisms in liquid chromatography with hydrophobic stationary phase. Anal Chem 86(10):4919–4926
Zhong DD, Huang YP, Xin XL, Liu ZS, Aisa HA (2013) Preparation of metallic pivot-based imprinted monolith for polar template. J Chromatogr B 934(1):109–116
Bian M, Zhang Z, Yin H (2012) Effects and mechanism characterization of ionic liquids as mobile phase additives for the separation of matrine-type alkaloids by liquid chromatography. J Pharm Biomed 58(25):163–167
Zhang YG, Song D, Lanni L, Shimizu K (2010) Importance of functional monomer dimerization in the molecular imprinting process. Macromolecules 43(15):6284–6294
Caro E, Masque N, Mzcre RM, Borrul F, Comack PAG, Sherrington DC (2002) Non-covalent and semi-covalent molecularly imprinted polymers for selective on-line solid-phase extraction of 4-nitrophenol from water samples. J Chromatogr A 963(1–2):169–178
Yang CX, Liu SS, Wang HF, Wang SW, Yan XP (2012) High-performance liquid chromatographic separation of position isomers using metal-organic framework MIL-53(Al) as the stationary phase. Analyst 137(1):133–139
Kaszynska MS, Jaszczolt K, Witt D, Rachon J (2004) High-performance liquid chromatography of di- and trisubstituted aromatic positional isomers on 1,3-alternate 25,27-dipropoxy-26,28-bis-[3-propyloxy]-calix[4] arene-bonded silica gel stationary phase. J Chromatogr A 1055(1–2):21–28
Sellergren B, Shea KJ (1995) Origin of peak asymmetry and the effect of temperature on solute retention in enantiomer separations on imprinted chiral stationary phases. J Chromatogr A 690(1):29–39
Denderz N, Lehotay J, Cizmarik J, Cibulkova Z, Simon P (2012) Thermodynamic study of molecularly imprinted polymer used as the stationary phase in high performance liquid chromatography. J Chromatogr A 1235(27):77–83
Dender N, Lehotay J (2012) Application of the Van’t Hoff dependences in the characterization of molecularly imprinted polymers for some phenolic acids. J Chromatogr A 1268(14):44–52
Kim H, Kaczmarski K, Guiochon G (2006) Thermodynamic analysis of the heterogeneous binding sites of molecularly imprinted polymers. J Chromatogr A 1101(1–2):136–152
Kim H, Guiochon G (2005) Thermodynamic functions and intraparticle mass transfer kinetics of structural analogues of a template on molecularly imprinted polymers in liquid chromatography. J Chromatogr A 1097(1–2):84–97
Liu YQ, Hoshina K, Haginaka J (2010) Monodispersed, molecularly imprinted polymers for cinchonidine by precipitation polymerization. Talanta 80(5):1713–1718
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21375096 and U1303202) and by the Hundred Talents Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 399 kb)
Rights and permissions
About this article
Cite this article
Wang, XH., Zhang, J., Peng, C. et al. Comparison of multi-recognition molecularly imprinted polymers for recognition of melamine, cyromazine, triamterene, and trimethoprim. Anal Bioanal Chem 407, 7145–7155 (2015). https://doi.org/10.1007/s00216-015-8878-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8878-9