Abstract
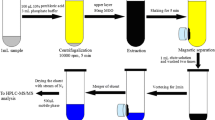
In this study, a simple solid-phase extraction (SPE) approach by using activated attapulgite as sorbent was successfully developed for the determination of melamine in milk formula samples. Crucial factors impacting the extraction efficiency, including sample solvent, elution solvent, and sample loading volume, were investigated. Under the optimal extraction conditions, the sample loading volume was up to 200 mL and the adsorption capacity of the melamine gave rise to 1154 μg g−1. Excellent linear calibration curves (r 2 > 0.999) were achieved, and then the limit of detection (S/N = 3) and the limit of quantification (S/N = 10) were found to be 0.15 and 0.5 ng mL−1, respectively. The recoveries of the melamine spiked in four milk formula samples at three concentration levels ranged from 83.5 to 111.0 % with relative standard deviations (RSDs) less than 10.2 %. Furthermore, RSDs of batch to batch (n = 4) of the acidified attapulgite used in this developed method were in the range of 2.3∼7.3 %. In comparison to the commercial Oasis MCX, the acidified attapulgite sorbent even outperformed (at least in terms of reproducibility) for melamine analysis in real food samples. Because of its simplicity, the newly developed SPE method based on acidified attapulgite nanoparticles should provide a promising tool for daily monitoring of doped melamine in milk formula or other complex matrices.





Similar content being viewed by others
References
Xu XM, Ren YP, Zhu Y, Cai ZX, Han JL, Huang BF, et al. Direct determination of melamine in dairy products by gas chromatography/mass spectrometry with coupled column separation. Anal Chim Acta. 2009;650:39–43.
Xia X, Ding S, Li X, Gong X, Zhang S, Jiang H, et al. Validation of a confirmatory method for the determination of melamine in egg by gas chromatography-mass spectrometry and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2009;651:196–00.
Sun H, Wang L, Ai L, Liang S, Wu H. A sensitive and validated method for determination of melamine residue in liquid milk by reversed phase high-performance liquid chromatography with solid-phase extraction. Food Control. 2010;21:686–91.
Zhang H, Zhang Z, Hu Y, Yang X, Yao S. Synthesis of a novel composite imprinted material based on multiwalled carbon nanotubes as a selective melamine absorbent. J Agric Food Chem. 2011;59:1063–71.
Cheng W, Liu Z, Wang Y. Preparation and application of surface molecularly imprinted silica gel for selective extraction of melamine from milk samples. Talanta. 2013;116:396–02.
He L, Su Y, Zheng Y, Huang X, Wu L, Liu Y, et al. Novel cyromazine imprinted polymer applied to the solid-phase extraction of melamine from feed and milk samples. J Chromatogr A. 2009;1216:6196–03.
Wang T, Zhu Y, Ma J, Xuan R, Gao H, Liang Z, et al. Hydrophilic solid-phase extraction of melamine with ampholine-modified hybrid organic-inorganic silica material. J Sep Sci. 2015;38:87–92.
Zhang Y, Lin S, Jiang P, Zhu X, Ling J, Zhang W, et al. Determination of melamine and cyromazine in milk by high performance liquid chromatography coupled with online solid-phase extraction using a novel cation-exchange restricted access material synthesized by surface initiated atom transfer radical polymerization. J Chromatogr A. 2014;1337:17–21.
Chen L, Liang H, Lu Y, Cui C, Yu S. Synthesis of an attapulgite clay@carbon nanocomposite adsorbent by a hydrothermal carbonization process and their application in the removal of toxic metal ions from water. Langmuir. 2011;27:8998–04.
Fan Q, Shao D, Lu Y, Wu W, Wang X. Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni(II) to Na-attapulgite. Chem Eng J. 2009;150:188–95.
Chen H, Zhao Y, Wang A. Removal of Cu(II) from aqueous solution by adsorption onto acid-activated palygorskite. J Hazard Mater. 2007;149:346–54.
Xue A, Zhou S, Zhao Y, Lu X, Han P. Effective NH2-grafting on attapulgite surfaces for adsorption of reactive dyes. J Hazard Mater. 2011;194:7–14.
Zhao Y, Chen Y, Li M, Zhou S, Xue A, Xing W. Adsorption of Hg2+ from aqueous solution onto polyacrylamide/attapulgite. J Hazard Mater. 2009;171:640–6.
Zhao C, Zhao T, Liu X, Zhang H. A novel molecularly imprinted polymer for simultaneous extraction and determination of sudan dyes by on-line solid phase extraction and high performance liquid chromatography. J Chromatogr A. 2010;1217:6995–02.
Zhao C, Guan X, Liu X, Zhang H. Synthesis of molecularly imprinted polymer using attapulgite as matrix by ultrasonic irradiation for simultaneous on-line solid phase extraction and high performance liquid chromatography determination of four estrogens. J Chromatogr A. 2012;1229:72–8.
Chu G, Cai W, Shao X. Preparation of 4-butylaniline-bonded attapulgite for pre-concentration of bisphenol A in trace quantity. Talanta. 2015;136:29–34.
Zhang L, Li Z, Hu Z, Chang X. Solid phase extraction of gold(III) on attapulgite modified with triocarbohydrazide prior to its determination in environmental samples by ICP-OES. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79:1234–9.
Zang Z, Li Z, Zhang L, Li R, Hu Z, Chang X, et al. Chemically modified attapulgite with asparagine for selective solid-phase extraction and preconcentration of Fe(III) from environmental samples. Anal Chim Acta. 2010;663:213–7.
Myriam M, Suárez M, Martín-Pozas JM. Structural and textural modifications of palygorskite and sepiolite under acid treatment. Clays Clay Miner. 1998;46:225–31.
Wang W, Chen H, Wang A. Adsorption characteristics of Cd(II) from aqueous solution onto activated palygorskite. Sep Purif Technol. 2007;55:157–64.
Wang T, Chen Y, Ma J, Qian Q, Jin Z, Zhang L, et al. Attapulgite nanoparticles-modified monolithic column for hydrophilic in-tube solid-phase microextraction of cyromazine and melamine. Anal Chem. 2016;88:1535–41.
Jandera P. Stationary and mobile phases in hydrophilic interaction chromatography: a review. Anal Chim Acta. 2011;692:1–25.
Guo Y, Gaiki S. Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J Chromatogr A. 2005;1074:71–80.
Horvath J, Dolník V. Polymer wall coating for capillary electrophoresis. Electrophoresis. 2001;22:644–55.
Dai H, Shi Y, Wang Y, Sun Y, Hu J, Ni P, et al. A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sensor Actuat B-Chem. 2014;202:201–8.
Boudriche L, Calvet R, Chamayou A, Hamdi B, Balard H. Removal of lead(II) from aqueous solution using modified palygorskite, contribution of inverse gas chromatography. J Chromatogr A. 2015;1408:207–16.
Tian M, Bi W, Row KH. Molecular imprinting in ionic liquid-modified porous polymer for recognitive separation of three tanshinones from Salvia miltiorrhiza Bunge. Anal Bioanal Chem. 2011;399:2495–02.
Su R, Wang X, Xu X, Wang Z, Li D, Zhao X, et al. Application of multiwall carbon nanotubes-based matrix solid phase dispersion extraction for determination of hormones in butter by gas chromatography mass spectrometry. J Chromatogr A. 2011;1218:5047–54.
Basheer C, Chong HG, Hii TM, Lee HK. Application of porous membrane-protected micro-solid-phase extraction combined with HPLC for the analysis of acidic drugs in waste water. Anal Chem. 2007;79:6845–50.
Acknowledgments
The project is supported by the National Natural Science Foundation of China (21405085), the Public Applied Research Programs of Technology of Zhejiang Province (2015C37015), the Zhejiang Provincial Natural Science Foundation of China (LQ12B05001), and Ningbo Natural Science Foundation of China (2012A610091).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Wang, TT., Xuan, RR., Ma, JF. et al. Using activated attapulgite as sorbent for solid-phase extraction of melamine in milk formula samples. Anal Bioanal Chem 408, 6671–6677 (2016). https://doi.org/10.1007/s00216-016-9779-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9779-2