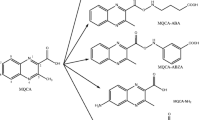
Abstract
The study of an enzyme-linked immunosorbent assay (ELISA) and an amperometric biosensor for the detection of the pyrethroid deltamethrin in seawater is reported. The preparation of specific polyclonal antibodies is addressed using two immunizing haptens based on deltamethrin and cypermethrin compounds, with a spacer arm placed at the cyano residue in the pyrethroid structure. Different conjugates based on bovine serum albumin and aminodextran are prepared depending on the lipophilic profile of the competitor haptens studied. A reproducible and sensitive indirect competitive ELISA is developed, reaching a limit of detection of 1.2 ± 0.04 μg L−1 and an IC50 value of 21.4 ± 0.3 μg L−1 (both n = 3). For validation of the assays described, artificial seawater samples fortified with deltamethrin are analyzed. For the ELISA assay, these accuracy studies reported a slope of 0.904. An amperometric immunosensor is developed using the same immunoreagents and achieving a comparable detectability in terms of LOD of 4.7 μg L−1, measuring seawater without any pretreatment. These results suggest that both techniques can be used as rapid and simple analytical methods for deltamethrin quantification in seawater samples, which are great candidates for initial environmental screening programs.

ᅟ





Similar content being viewed by others
References
Mak SK, Shan G, Lee H-J, Watanabe T, Stoutamire DW, Gee SJ, et al. Development of a class selective immunoassay for the type II pyrethroid insecticides. Anal Chim Acta. 2005;534(1):109–20. https://doi.org/10.1016/j.aca.2004.11.021.
Shan G, Wengatz I, Stoutamire DW, Gee SJ, Hammock BD. An enzyme-linked immunosorbent assay for the detection of esfenvalerate metabolites in human urine. Chem Res Toxicol. 1999;12:1033–41.
Lee H-J, Shan G, Watanabe T, Stoutamire DW, Gee SJ, Hammock BD. Enzyme-linked immunosorbent assay for the pyrethroid deltamethrin. J Agric Food Chem. 2002;50:5526–32.
Taheri N, Lan M, Wei P, Liu R, Gui W, Guo Y, et al. Chemiluminescent enzyme immunoassay for rapid detection of three α-cyano pyrethroid residues in agricultural products. Food Anal Methods. 2016;9(10):2896–905. https://doi.org/10.1007/s12161-016-0482-x.
Hernandes T, Dores EFGC, Ribeiro ML, Rossignoli PA, Malm O. Simple method to determine residual cypermethrin and deltamethrin in bovine milk. J Braz Chem Soc. 2014;25(9):1656–61. https://doi.org/10.5935/0103-5053.20140154.
Palma DCA, Lourencetti C, Uecker ME, Mello PRB, Pignati WA, Dores EFGC. Simultaneous determination of different classes of pesticides in breast milk by solid-phase dispersion and GC/ECD. J Braz Chem Soc. 2014;25(8):1419–30. https://doi.org/10.5935/0103-5053.20140124.
Hao XL, Kuang H, Li YL, Yuan Y, Peng CF, Chen W, et al. Development of an enzyme-linked immunosorbent assay for the alpha-cyano pyrethroids multiresidue in Tai Lake water. J Agric Food Chem. 2009;57:3033–9.
Moschet C, Vermeirssen EL, Seiz R, Pfefferli H, Hollender J. Picogram per liter detections of pyrethroids and organophosphates in surface waters using passive sampling. Water Res. 2014;66:411–22. https://doi.org/10.1016/j.watres.2014.08.032.
Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30(2):55–78. https://doi.org/10.1016/j.ntt.2007.10.005.
Laranjeiro F, Sánchez-Marín P, Galante-Oliveira S, Barroso C. Tributyltin pollution biomonitoring under the Water Framework Directive: proposal of a multi-species tool to assess the ecological quality status of EU water bodies. Ecol Indic. 2015;57:525–35. https://doi.org/10.1016/j.ecolind.2015.04.039.
Shephard S, Greenstreet SPR, Piet GJ, Rindorf A, Dickey-Collas M. Surveillance indicators and their use in implementation of the Marine Strategy Framework Directive. ICES J Mar Sci. 2015;72(8):2269–77. https://doi.org/10.1093/icesjms/fsv131.
Esteve-Turrillas FA, Pastor A, Guardia MDL. Determination of pyrethroid insecticide residues in vegetable oils by using combined solid-phases extraction and tandem mass spectrometry detection. Anal Chim Acta. 2005;553(1–2):50–7. https://doi.org/10.1016/j.aca.2005.08.004.
Ferrer C, Gómez MJ, García-Reyes JF, Ferrer I, Thurman EM, Fernández-Alba AR. Determination of pesticide residues in olives and olive oil by matrix solid-phase dispersion followed by gas chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. J Chromatogr A. 2005;1069(2):183–94. https://doi.org/10.1016/j.chroma.2005.02.015.
Pavan FA, Dallago RM, Zanella R, Martins AF. Determination of deltamethrin in cattle dipping baths by high-performance liquid chromatography. J Agric Food Chem. 1999;47:174–6.
Boonchiangma S, Ngeontae W, Srijaranai S. Determination of six pyrethroid insecticides in fruit juice samples using dispersive liquid-liquid microextraction combined with high performance liquid chromatography. Talanta. 2012;88:209–15. https://doi.org/10.1016/j.talanta.2011.10.033.
Hu L, Wang X, Qian H, Wang H, Lu R, Zhang S, et al. In-syringe low-density ionic liquid dispersive liquid–liquid microextraction for the fast determination of pyrethroid insecticides in environmental water samples by HPLC-DAD. RSC Adv. 2016;6(73):69218–25. https://doi.org/10.1039/c6ra09668a.
Mukdasai S, Thomas C, Srijaranai S. Two-step microextraction combined with high performance liquid chromatographic analysis of pyrethroids in water and vegetable samples. Talanta. 2014;120:289–96. https://doi.org/10.1016/j.talanta.2013.12.005.
López-López T, Gil-Garcia MD, Martínez-Vidal JL, Martínez-Galera M. Determination of pyrethroids in vegetables by HPLC using continuous on-line post-elution photoirradiation with fluorescence detection. Anal Chim Acta. 2001;447:101–11.
Vazquez PP, Mughari AR, Galera MM. Solid-phase microextraction (SPME) for the determination of pyrethroids in cucumber and watermelon using liquid chromatography combined with post-column photochemically induced fluorimetry derivatization and fluorescence detection. Anal Chim Acta. 2008;607(1):74–82. https://doi.org/10.1016/j.aca.2007.11.027.
Feo ML, Ginebreda A, Eljarrat E, Barceló D. Presence of pyrethroid pesticides in water and sediments of Ebro River Delta. J Hydrol. 2010;393(3–4):156–62. https://doi.org/10.1016/j.jhydrol.2010.08.012.
Shirani M, Haddadi H, Rezaee M, Semnani A, Habibollahi S. Solid-phase extraction combined with dispersive liquid–liquid microextraction for the simultaneous determination of deltamethrin and permethrin in honey by gas chromatography–mass spectrometry. Food Anal Methods. 2016;9(9):2613–20. https://doi.org/10.1007/s12161-016-0455-0.
Ahn KC, Kim HJ, McCoy MR, Gee SJ, Hammock BD. Immunoassays and biosensors for monitoring environmental and human exposure to pyrethroid insecticides. J Agric Food Chem. 2011;59(7):2792–802. https://doi.org/10.1021/jf1033569.
Kong Y, Zhang Q, Zhang W, Gee SJ, Li P. Development of a monoclonal antibody-based enzyme immunoassay for the pyrethroid insecticide deltamethrin. J Agric Food Chem. 2010;58(14):8189–95. https://doi.org/10.1021/jf101483w.
Lee H-J, Shan G, Ahn KC, Park E-K, Watanabe T, Gee SJ, et al. Development of an enzyme-linked immunosorbent assay for the pyrethroid cypermethrin. J Agric Food Chem. 2004;52(5):1039–43. https://doi.org/10.1021/jf030519p.
Queffelec A-L, Nodet P, Haelters J-P, Thouvenot D, Corbel B. Hapten synthesis for a monoclonal antibody based ELISA for deltamethrin. J Agric Food Chem. 1998;46(4):1670–6. https://doi.org/10.1021/jf9703922.
Zhang Z, Zeng K, Liu J. Immunochemical detection of emerging organic contaminants in environmental waters. TrAC Trends Anal Chem. 2017;87:49–57. https://doi.org/10.1016/j.trac.2016.12.002.
Ge S, Zhang C, Yu F, Yan M, Yu J. Layer-by-layer self-assembly CdTe quantum dots and molecularly imprinted polymers modified chemiluminescence sensor for deltamethrin detection. Sensors Actuators B Chem. 2011;156(1):222–7. https://doi.org/10.1016/j.snb.2011.04.024.
Abegao LM, Ribeiro JH, Ribeiro PA, Raposo M. Nano-molar deltamethrin sensor based on electrical impedance of PAH/PAZO layer-by-layer sensing films. Sensors (Basel). 2013;13(8):10167–76. https://doi.org/10.3390/s130810167.
Liu X, Li L, Liu YQ, Shi XB, Li WJ, Yang Y, et al. Ultrasensitive detection of deltamethrin by immune magnetic nanoparticles separation coupled with surface plasmon resonance sensor. Biosens Bioelectron. 2014;59:328–34. https://doi.org/10.1016/j.bios.2014.03.020.
Castellarnau M, Ramon-Azcon J, Gonzalez-Quinteiro Y, Lopez JF, Grimalt JO, Marco MP, et al. Assessment of analytical methods to determine pyrethroids content of bednets. Tropical Med Int Health. 2017;22(1):41–51. https://doi.org/10.1111/tmi.12794.
Jiang X, Li D, Xu X, Ying Y, Li Y, Ye Z, et al. Immunosensors for detection of pesticide residues. Biosens Bioelectron. 2008;23(11):1577–87. https://doi.org/10.1016/j.bios.2008.01.035.
Salvador JP, Sanchez-Baeza F, Marco MP. Preparation of antibodies for the designer steroid tetrahydrogestrinone and development of an enzyme-linked immunosorbent assay for human urine analysis. Anal Chem. 2007;79(10):3734–40.
Salvador JP, Marco MP. Amperometric biosensor for continuous monitoring Irgarol 1051 in sea water. Electroanalysis. 2016;28(8):1833–8. https://doi.org/10.1002/elan.201600172.
Lee N, McAdam DP, Skerrit JH. Development of immunoassays for type II synthetic pyrethroids. 1. Hapten design and application to heterologous and homologous assays. J Agric Food Chem. 1998;46:520–34.
Sanborn JR, Gee SJ, Gilman SD, Sugawara Y, Jones AD, Rogers J, et al. Hapten synthesis and antibody development for polychlorinated dibenzo-p-dioxin immunoassays. J Agric Food Chem. 1998;46:2407–16.
Estévez MC, Kreuzer M, Sánchez-Baeza F, Marco MP. Analysis of nonylphenol: advances and improvements in the immunochemical determination using antibodies raised against the technical mixture and hydrophilic immunoreagents. Environ Sci Technol. 2006;40(2):559–68. https://doi.org/10.1021/es050984y.
Galve R, Nichkova M, Camps F, Sanchez-Baeza F, Marco MP. Development and evaluation of an immunoassay for biological monitoring chlorophenols in urine as potential indicators of occupational exposure. Anal Chem. 2002;74:468–78.
Sanvicens N, Varela B, Ballesteros B, Marco MP. Development of an immunoassay for terbutryn: study of the influence of the immunization protocol. Talanta. 2012;89:310–6. https://doi.org/10.1016/j.talanta.2011.12.033.
Nichkova M, Gemani M, Marco MP. Immunochemical analysis of 2,4,6-tribromophenol for assessment of wood contamination. J Agric Food Chem. 2008;56:29–34.
Abad L, Javier del Campo F, Muñoz FX, Fernández LJ, Calavia D, Colom G, et al. Design and fabrication of a COP-based microfluidic chip: chronoamperometric detection of Troponin T. Electrophoresis. 2012;33(21):3187–94. https://doi.org/10.1002/elps.201200225.
Fernandez F, Hegnerova K, Piliarik M, Sanchez-Baeza F, Homola J, Marco MP. A label-free and portable multichannel surface plasmon resonance immunosensor for on site analysis of antibiotics in milk samples. Biosens Bioelectron. 2010;26(4):1231–8. https://doi.org/10.1016/j.bios.2010.06.012.
Esteban-Fernández de Ávila B, Escamilla-Gómez V, Campuzano S, Pedrero M, Pingarrón JM. Disposable electrochemical magnetoimmunosensor for the determination of Troponin T cardiac marker. Electroanalysis. 2013;25(1):51–8. https://doi.org/10.1002/elan.201200250.
Funding
This work has been funded by SEA-on-a-CHIP project (FP7-OCEAN-2013, no 614168). The Nb4D group (formerly Applied Molecular Receptors group, AMRg) is a consolidated research group (Grup de Recerca) of the Generalitat de Catalunya and has support from the Departament d’Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya (expedient: 2014 SGR 1484). CIBER-BBN is an initiative funded by the Spanish National Plan for Scientific and Technical Research and Innovation 2013–2016; Iniciativa Ingenio 2010, Consolider Program, and CIBER Actions are financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund. The ICTS “NANOBIOSIS,” and particularly the Custom Antibody Service (CAbS, IQAC-CSIC, CIBER-BBN), is acknowledged for the assistance and support related to the immunoreagents used in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Rabbit immunizations included in this study were carried out in the animal facility of the Research and Development Center (CID) from the Spanish Research Council (CSIC) -Registration Number B9900083. All efforts were made to minimize suffering of the animals. The protocol used for the production of antibodies was conducted in accordance with the institutional guidelines under a license from the local government (DAAM 7463) and was approved by the Institutional Animal Care and Use Committee at the CID-CSIC.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 247 kb)
Rights and permissions
About this article
Cite this article
Fruhmann, P., Sanchis, A., Mayerhuber, L. et al. Immunoassay and amperometric biosensor approaches for the detection of deltamethrin in seawater. Anal Bioanal Chem 410, 5923–5930 (2018). https://doi.org/10.1007/s00216-018-1209-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1209-1