Abstract
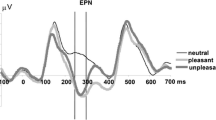
Event-related potentials were recorded from 30 subjects using sustained stimulation and an indirect task, two strategies which facilitate affective responses that are complete and free of cognitive interference. Stimuli were of three types: pleasant, unpleasant and neutral. A three-phase pattern was found. The first phase, an amplitude increase in response to negative stimuli higher than to neutral and pleasant stimuli, was produced at 160 ms after stimulus onset, the prefrontal cortex being the origin of this phase. The second phase, characterized by maximal amplitudes in response to positive stimuli, was produced at 400 ms, originating in the visual cortex. Finally, the third phase, another amplitude increase in response to negative stimuli, was produced at 680 ms, and its source was located in the left precentral gyrus. Present data show that the cortical response to sustained emotional visual stimulation presented within indirect tasks provides information on attention-, motivation- and motor-related biases that complement information obtained under other experimental conditions.




Similar content being viewed by others
References
Armony JL, Dolan RJ (2002) Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia 40:817–826
Bar M (2003) A cortical mechanism for triggering top–down facilitation in visual object recognition. J Cogn Neurosci 15:600–609
Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmidt AM, Dale AM, Hämäläinen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E (2006) Top–down facilitation of visual recognition. Proc Natl Acad Sci USA 103:449–454
Cacioppo JT, Gardner WL (1999) Emotion. Annu Rev Psychol 50:191–214
Caldara R, Deiber MP, Andrey C, Michel CM, Thut G, Hauert CA (2004) Actual and mental motor preparation and execution: a spatiotemporal ERP study. Exp Brain Res 159:389–399
Carretié L, Iglesias J, García T, Ballesteros M (1997) N300, P300 and the emotional processing of visual stimuli. Electroencephalogr Clin Neurophysiol 103:298–303
Carretié L, Martín-Loeches M, Hinojosa JA, Mercado F (2001): Attention and emotion interaction studied through event related potentials. J Cogn Neurosci 13:1109–1128
Carretié L, Hinojosa JA, Mercado F (2003) Cerebral patterns of attentional habituation to emotional visual stimuli. Psychophysiology 40:381–388
Carretié L, Hinojosa JA, Martín-Loeches M, Mercado F, Tapia M (2004a) Automatic attention to emotional stimuli: neural correlates. Hum Brain Mapp 22:290–299
Carretié L, Tapia M, Mercado F, Albert J, López-Martín S, de la Serna JM (2004b) Voltage-based versus factor score-based source localization analyses of electrophysiological brain activity: a comparison. Brain Topogr 17:109–115
Carretié L, Hinojosa JA, Mercado F, Tapia M (2005) Cortical response to subjectively unconscious danger. Neuroimage 26:615–623
Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F (2000) The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex 10:220–242
Chapman RM, McCrary JW (1995) EP component identification and measurement by principal components analysis. Brain Cogn 27:288–310
Cliff N (1987) Analyzing multivariate data. Harcourt Brace Jovanovich, New York
Coles MGH, Gratton G, Kramer AF, Miller GA (1986) Principles of signal acquisition and analysis. In: Coles MGH, Donchin E, Porges SW (eds) Psychophysiology: systems, processes and applications. Elsevier, Amsterdam pp 183–221
Coombes SA Cauraugh JH, Janelle CM (2006) Emotion and movement: activation of defensive circuitry alters the magnitude of a sustained muscle contraction. Neurosci Lett 396:192–196
Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ (2000) Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol 52:95–111
Delplanque S, Lavoie ME, Hot P, Silvert L, Sequeira H (2004) Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neurosci Lett 356:1–4
Dien J, Beal D J, Berg P (2005) Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clin Neurophysiol 116:1808–1825
Dierks T, Jelic V, Pascual-Marqui RD, Wahlund L, Julin P, Linden DE, Maurer K, Winblad B, Nordberg A (2000) Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG-generators in Alzheimer’s disease. Clin Neurophysiol 111:1817–1824
Donchin E, Heffley EF (1978) Multivariate analysis of event-related potential data: a tutorial review. In: Otto D (ed) Multidisciplinary perspectives in event related brain potential research. U.S. Government Printing Office, Washington, DC pp 555–572
Duncan-Johnson CC, Donchin E (1977) On quantifying surprise: the variation of event-related potentials with subjective probability. Psychophysiology 14:456–467
Fabiani M, Gratton G, Karis D, Donchin E (1987) Definition, identification, and reliability of measurement of the P300 component of the event related brain potential. In: Acles PK, Jennings JR, Coles MGH (eds) Advances in psychophysiology, vol. 2. JAI Press, London pp 1–78
Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K (2000) Facial expressions of emotion: are angry faces detected more efficiently? Cogn Emot 14:61–92
Hansen CH, Hansen RD (1988) Finding the face in the crowd: an anger superiority effect. J Pers Soc Psychol 54:917–924
Hopfinger JB, Buonocore MH, Mangun GR (2000) The neural mechanisms of top–down attentional control. Nat Neurosci 3:284–291
Huettel SA, McKeown MJ, Song AW, Hart S, Spencer DD, Allison T, McCarthy G (2004) Linking hemodynamic and electrophysiological measures of brain activity: evidence from functional MRI and intracranial field potentials. Cereb Cortex 14:165–173
Kawasaki H, Adolphs R, Kaufman O, Damasio H, Damasio AR, Granner M, Bakken H, Hori T, Howard MA (2001) Single neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat Neurosci 4:15–16
Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang P (2002) Large-scale neural correlates of affective picture processing. Psychophysiology 39:641–649
Lang PJ, Greenwald MK, Bradley MM, Hamm AO (1993): Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 30:261–273
Lang PJ, Bradley MM, Cuthbert BN (2001) International affective picture system (IAPS): Instruction manual and affective ratings. Technical report A-5. The Center for Research in Psychophysiology, University of Florida, Gainesville, FL
Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, Kaulisch T, Kötter R, Stephan KE, Leschinger A, Hagner T, Bargel B, Witzel T, Hinrichs H, Bogerts B, Scheich H, Heinze HJ (2000) Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cereb Cortex 10:93–107
Ohman A, Flykt A, Esteves F (2001) Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen 130:466–478
Osgood C, Suci G, Tannenbaum P (1957) The measurement of meaning. University of Illinois, Urbana, IL
Pahlavan F, Duda D, Bonnet P (2000) Direction of human motor response by men and women to aversive stimulation. Percept Mot Skills 90:415–422
Pascual-Marqui RD (1999) Review of methods for solving the EEG inverse problem. Int J Bioelectromagn 1:75–86
Pascual-Marqui RD, Michel CM, Lehman D (1994) Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18:49–65
Pourtois G, Grandjean D, Sander D, Vuilleumier P (2004) Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb Cortex 14:619–633
Rodríguez M, Muñiz R, González B, Sabaté M (2004) Hand movement distribution in the motor cortex: the influence of a concurrent task and motor imagery. NeuroImage 22:1480–1491
Russell JA (1979) Affective space is bipolar. J Pers Soc Psychol 37:345–356
Sarter M, Givens B, Bruno JP (2001) The cognitive neuroscience of sustained attention: where top–down meets bottom up. Brain Res Rev 35:146–160
Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ (2000) Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37:257–261
Schupp HT, Junghöfer M, Weike AI, Hamm AO (2003) Emotional facilitation of sensory processing in the visual cortex. Psychol Sci 14:7–13
Simmons WK, Martin A, Barsalou LW (2005) Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex 15:1602–1608
Smith CA, Ellsworth PC (1985) Patterns of cognitive appraisal in emotion. J Pers Soc Psychol 48:813–838
Sokolov EN (1963) Perception and the conditioned reflex. Pergamon Press, Oxford
Spencer KM, Dien J, Donchin E (1999) A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology 36:409–414
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. Thieme, Stuttgart
Taylor SE (1991) Asymmetrical effects of positive and negative events. The mobilization–immobilization hypothesis. Psychol Bull 110:67–85
Vitacco D, Brandeis D, Pascual-Marqui RD, Martín E (2002) Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum Brain Mapp 17:4–12
Acknowledgments
This work was supported by the grants BSO2002-01980 from the Ministerio de Educación y Ciencia of Spain and 06/HSE/0032/2004 from the Communidad de Madrid. A part of the data described in this article was presented at the IX International Conference on Cognitive Neuroscience (ICON 9; Havana, Cuba, September 2005).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
List of the stimuli used in the present experiment, along with their values in Valence (from 1, highly unpleasant, to 9, highly pleasant) and Arousal (from 1, highly relaxing, to 9, highly arousing) as provided by the International Affective Picture System (IAPS; Lang et al. 2001). Stimuli written in italics contained a person, those in regular letters did not (see the text)
Negative | Neutral | Positive | ||||||
|---|---|---|---|---|---|---|---|---|
Stimulus (IAPS code) | Valence | Arousal | Stimulus (IAPS code) | Valence | Arousal | Stimulus (IAPS code) | Valence | Arousal |
1201 | 3.55 | 6.36 | 2235 | 5.64 | 3.36 | 4659 | 6.87 | 6.93 |
2095 | 1.79 | 5.25 | 2372 | 5.48 | 4.09 | 4669 | 5.97 | 6.11 |
6550 | 2.73 | 7.09 | 2383 | 4.72 | 3.41 | 4687 | 6.87 | 6.51 |
8580 | 3.7 | 6.28 | 2393 | 4.87 | 2.93 | 4800 | 6.44 | 7.07 |
1050 | 3.46 | 6.87 | 7009 | 4.93 | 3.01 | 5480 | 7.53 | 5.48 |
1275 | 3.3 | 4.81 | 7025 | 4.63 | 2.71 | 7230 | 7.38 | 5.52 |
1300 | 3.55 | 6.79 | 7041 | 4.99 | 2.6 | 7270 | 7.53 | 5.76 |
9570 | 1.68 | 6.14 | 7100 | 5.24 | 2.89 | 7289 | 6.32 | 5.14 |
Means | 2.97 | 6.20 | 5.06 | 3.13 | 6.44 | 6.09 | ||
Rights and permissions
About this article
Cite this article
Carretié, L., Hinojosa, J.A., Albert, J. et al. Neural response to sustained affective visual stimulation using an indirect task. Exp Brain Res 174, 630–637 (2006). https://doi.org/10.1007/s00221-006-0510-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0510-y