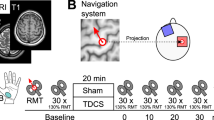
Abstract
Transcranial direct current stimulation (tDCS) uses a weak electric current to modulate neuronal activity. A neurophysiologic outcome measure to demonstrate reliable tDCS modulation at the group level is transcranial magnetic stimulation engendered motor evoked potentials (MEPs). Here, we conduct a study testing the reliability of individual MEP response patterns following a common tDCS protocol. Fourteen participants (7m/7f) each underwent nine randomized sessions of 1 mA, 10 min tDCS (3 anode; 3 cathode; 3 sham) delivered using an M1/orbito-frontal electrode montage (sessions separated by an average of ~5.5 days). Fifteen MEPs were obtained prior to, immediately following and in 5 min intervals for 30 min following tDCS. TMS was delivered at 130 % resting motor threshold using neuronavigation to ensure consistent coil localization. A number of non-experimental variables were collected during each session. At the individual level, considerable variability was seen among different testing sessions. No participant demonstrated an excitatory response ≥20 % to all three anodal sessions, and no participant demonstrated an inhibitory response ≥20 % to all three cathodal sessions. Intra-class correlation revealed poor anodal and cathodal test–retest reliability [anode: ICC(2,1) = 0.062; cathode: ICC(2,1) = 0.055] and moderate sham test–retest reliability [ICC(2,1) = 0.433]. Results also revealed no significant effect of tDCS at the group level. Using this common protocol, we found the effects of tDCS on MEP amplitudes to be highly variable at the individual level. In addition, no significant effects of tDCS on MEP amplitude were found at the group level. Future studies should consider utilizing a more strict experimental protocol to potentially account for intra-individual response variations.







Similar content being viewed by others
References
Amassian VE, Cracco RQ (1987) Human cerebral cortical responses to contralateral transcranial stimulation. Neurosurgery 20(1):148–155
Amassian VE, Cracco RQ, Maccabee PJ (1989) Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol 74(6):401–416
Ardolino G, Bossi B, Barbieri S, Priori A (2005) Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol 568(Pt 2):653–663. doi:10.1113/jphysiol.2005.088310
Bashir S, Perez J, Horvath JC, Pascual-Leone A (2013) Differentiation of motor cortical representation of hand muscles by navigated mapping of optimal TMS current directions in healthy subjects. J Clin Neurophysiol 30(4):390
Bastani A, Jaberzadeh S (2012) A higher number of TMS-elicited MEP from a combined hotspot improves intra-and inter-session reliability of the upper limb muscles in healthy individuals. PLoS One 7(10):e47582
Bastani A, Jaberzadeh S (2013) Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PLoS One 8(8):e72254
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA (2013) Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591(7):1987–2000
Bikson M, Datta A, Rahman A, Scaturro J (2010) Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clin Neurophysiol 121(12):1976
Bradnam LV, Stinear CM, Byblow WD (2011) Cathodal transcranial direct current stimulation suppresses ipsilateral projections to presumed propriospinal neurons of the proximal upper limb. J Neurophysiol 105(5):2582–2589
Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG (1992) Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol 85(1):9–16
Chaieb L, Antal A, Paulus W (2008) Gender-specific modulation of short-term neuroplasticity in the visual cortex induced by transcranial direct current stimulation. Vis Neurosci 25(01):77–81
Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim YH, Pascual-Leone A (2016) Optimal number of pulses as outcome measure in neuronavigated transcranial magnetic stimulation. Clin Neurophysiol [Epub ahead of print]
Chew T, Ho KA, Loo CK (2015) Inter- and intra-individual variability in response to transcranial direct current stimulation(tDCS) at varying current intensities. Brain Stimul 8(6):1130–1137
Datta A, Baker JM, Bikson M, Fridriksson J (2011) Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul 4(3):169–174
de Tommaso M, Invitto S, Ricci K, Lucchese V, Delussi M, Quattromini P et al (2014) Effects of anodal TDCS stimulation of left parietal cortex on visual spatial attention tasks in men and women across menstrual cycle. Neurosci Lett 574:21–25. doi:10.1016/j.neulet.2014.05.014
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P et al (2004) The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115(2):255–266
Di Lazzaro V, Manganelli F, Dileone M, Notturno F, Esposito M, Capasso M et al (2012) The effects of prolonged cathodal direct current stimulation on the excitatory and inhibitory circuits of the ipsilateral and contralateral motor cortex. J Neural Transm 119(12):1499–1506. doi:10.1007/s00702-012-0845-4
Ellaway PH, Davey NJ, Maskill DW, Rawlinson SR, Lewis HS, Anissimova NP (1998) Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr Clin Neurophysiol 109(2):104–113
Fleiss JL (1986) The design and analysis of clinical experiments. Wiley, New York
Fujiyama H, Hyde J, Hinder MR, Kim SJ, McCormack GH, Vickers JC, Summers JJ (2014) Delayed plastic responses to anodal tDCS in older adults. Front Aging Neurosci 6:10–3389
Guggisberg AG, Dubach P, Hess CW, Wüthrich C, Mathis J (2001) Motor evoked potentials from masseter muscle induced by transcranial magnetic stimulation of the pyramidal tract: the importance of coil orientation. Clin Neurophysiol 112(12):2312–2319
Hahn C, Rice J, Macuff S, Minhas P, Rahman A, Bikson M (2013) Methods for extra-low voltage transcranial direct current stimulation: current and time dependent impedance decreases. Clin Neurophysiol 124(3):551–556
Horvath JC (2015) Are current blinding methods for transcranial direct current stimulation (tDCS) effective in healthy populations? Clin Neurophysiol 126(11):2045–2046
Horvath JC, Carter O, Forte JD (2014) Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Front Syst Neurosci 8:2. doi:10.3389/fnsys.2014.00002
Horvath JC, Forte JD, Carter O (2015a) Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review. Neuropsychologia 66:213–236
Horvath JC, Forte JD, Carter O (2015b) Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimul 8(3):535–550
Jacobson L, Koslowsky M, Lavidor M (2012) tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res 216(1):1–10
Julkunen P, Säisänen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, Könönen M (2009) Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage 44(3):790–795
Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG (1998) The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci 95(3):861–868
Kasai T, Kawai S, Kawanishi M, Yahagi S (1997) Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res 744(1):147–150
Kiers L, Cros D, Chiappa KH, Fang J (1993) Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89(6):415–423
Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, Nitsche MA (2013) Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul 6(4):644–648
Labruna L, Jamil A, Fresnoza S, Batsikadze G, Kuo MF et al (2016) Efficacy of Anodal Transcranial Direct Current Stimulation is Related to Sensitivity to Transcranial Magnetic Stimulation. Brain Stimul 9:8–15
Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN (2004a) Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res 156(4):439–443. doi:10.1007/s00221-003-1800-2
Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004b) Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry 56(9):634–639
Lopez-Alonso V, Cheeran B, Rio-Rodriguez D, Fernandez-Del-Olmo M (2014) Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 7(3):372–380
López-Alonso V, Fernández-del-Olmo M, Costantini A, Gonzalez-Henriquez JJ, Cheeran B (2015) Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin Neurophysiol 126(12):2342–2347
Madhavan S, Stinear JW (2010) Focal and bi-directional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul 3(1):42
Miyaguchi S, Onishi H, Kojima S, Sugawara K, Tsubaki A, Kirimoto H et al (2013) Corticomotor excitability induced by anodal transcranial direct current stimulation with and without non-exhaustive movement. Brain Res 1529:83–91. doi:10.1016/j.brainres.2013.07.026
Monte-Silva K, Kuo MF, Thirugnanasambandam N, Liebetanz D, Paulus W, Nitsche MA (2009) Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci 29(19):6124–6131
Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche MA (2010) Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J Neurophysiol 103(4):1735–1740
Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA (2013) Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6(3):424–432
Nielsen JF (1996) Improvement of amplitude variability of motor evoked potentials in multiple sclerosis patients and in healthy subjects. Electroencephalogr Clin Neurophysiol 101(5):404–411
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3):633–639
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57(10):1899–1901
Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W (2003) Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 114(4):600–604
Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N et al (2007) Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 97(4):3109–3117. doi:10.1152/jn.01312.2006
O’Connell NE, Cossar J, Marston L, Wand BM, Bunce D, Moseley GL, De Souza LH (2012) Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. PLoS One 7(10):e47514. doi:10.1371/journal.pone.0047514
Parkin BL, Ekhtiari H, Walsh VF (2015) Non-invasive human brain stimulation in cognitive neuroscience: a primer. Neuron 87(5):932–945
Pelletier SJ, Cicchetti F (2015) Cellular and molecular mechanisms of action of transcranial direct current stimulation: evidence from in vitro and in vivo models. Int J Neuropsychopharmacol 18(2):pyu047
Pellicciari MC, Brignani D, Miniussi C (2013) Excitability modulation of the motor system induced by transcranial direct current stimulation: a multimodal approach. Neuroimage 83:569–580. doi:10.1016/j.neuroimage.2013.06.076
Plowman-Prine EK, Triggs WJ, Malcolm MP, Rosenbek JC (2008) Reliability of transcranial magnetic stimulation for mapping swallowing musculature in the human motor cortex. Clin Neurophysiol 119(10):2298–2303
Pocock SJ, Assmann SE, Enos LE, Kasten LE (2002) Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 21:2917–2930
Power HA, Norton JA, Porter CL, Doyle Z, Hui I, Chan KM (2006) Transcranial direct current stimulation of the primary motor cortex affects cortical drive to human musculature as assessed by intermuscular coherence. J Physiol 577(Pt 3):795–803. doi:10.1113/jphysiol.2006.116939
Quartarone A, Morgante F, Bagnato S, Rizzo V, Sant’Angelo A, Aiello E et al (2004) Long lasting effects of transcranial direct current stimulation on motor imagery. NeuroReport 15(8):1287–1291
Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant’Angelo A, Romano M et al (2005) Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain 128(8):1943–1950
Roche N, Lackmy A, Achache V, Bussel B, Katz R (2011) Effects of anodal transcranial direct current stimulation over the leg motor area on lumbar spinal network excitability in healthy subjects. J Physiol 589(Pt 11):2813–2826. doi:10.1113/jphysiol.2011.205161
Rosler KM, Roth DM, Magistris MR (2008) Trial-to-trial size variability of motor-evoked potentials. A study using the triple stimulation technique. Exp Brain Res 187(1):51–59. doi:10.1007/s00221-008-1278-z
Rotenberg A, Horvath JC, Pascual-Leone A (2014) Transcranial magnetic stimulation. Springer, New York
Roy Choudhury K, Boyle L, Burke M, Lombard W, Ryan S, McNamara B (2011) Intra subject variation and correlation of motor potentials evoked by transcranial magnetic stimulation. Ir J Med Sci 180:873–880
Royal I, Lidji P, Théoret H, Russo FA, Peretz I (2015) Excitability of the motor system: a transcranial magnetic stimulation study on singing and speaking. Neuropsychologia 75:525–532
Russell M, Goodman T, Wang Q, Groshong B, Lyeth BG (2014) Gender differences in current received during transcranial electrical stimulation. Front Psychiatry 5:104. doi:10.3389/fpsyt.2014.00104
Scelzo E, Giannicola G, Rosa M, Ciocca M, Ardolino G, Cogiamanian F et al (2011) Increased short latency afferent inhibition after anodal transcranial direct current stimulation. Neurosci Lett 498(2):167–170. doi:10.1016/j.neulet.2011.05.007
Schabrun SM, Chipchase LS, Zipf N, Thickbroom GW, Hodges PW (2013) Interaction between simultaneously applied neuromodulatory interventions in humans. Brain Stimul 6(4):624–630
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24(13):3379–3385
Simis M, Adeyemo BO, Medeiros LF, Miraval F, Gagliardi RJ, Fregni F (2013) Motor cortex-induced plasticity by noninvasive brain stimulation: a comparison between transcranial direct current stimulation and transcranial magnetic stimulation. NeuroReport 24(17):973–975
Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM (1999) Menstrual cycle effects on cortical excitability. Neurology 53(9):2069–2072
Stagg CJ, Nitsche MA (2011) Physiological basis of transcranial direct current stimulation. Neuroscientist 17(1):37–53. doi:10.1177/1073858410386614
Stinear CM, Byblow WD (2003) Motor imagery of phasic thumb abduction temporally and spatially modulates corticospinal excitability. Clin Neurophysiol 114(5):909–914
Suzuki K, Fujiwara T, Tanaka N, Tsuji T, Masakado Y, Hase K et al (2012) Comparison of the after-effects of transcranial direct current stimulation over the motor cortex in patients with stroke and healthy volunteers. Int J Neurosci 122(11):675–681. doi:10.3109/00207454.2012.707715
Teo JT, Bentley G, Lawrence P, Soltesz F, Miller S, Wille D et al (2014) Late cortical plasticity in motor and auditory cortex: role of met-allele in BDNF Val66Met polymorphism. Int J Neuropsychopharmacol 17(5):705–713. doi:10.1017/S1461145713001636
Tremblay S, Beaule V, Lepage JF, Theoret H (2013) Anodal transcranial direct current stimulation modulates GABAB-related intracortical inhibition in the M1 of healthy individuals. NeuroReport 24(1):46–50. doi:10.1097/WNR.0b013e32835c36b8
Wassermann E, Epstein C, Ziemann U (2008) Oxford handbook of transcranial magnetic stimulation. Oxford University Press, Oxford
Wiethoff S, Hamada M, Rothwell JC (2014) Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 7(3):468–475. doi:10.1016/j.brs.2014.02.003
Wolf SL, Butler AJ, Campana GI, Parris TA, Struys DM, Weinstein SR, Weiss P (2004) Intra-subject reliability of parameters contributing to maps generated by transcranial magnetic stimulation in able-bodied adults. Clin Neurophysiol 115(8):1740–1747
Ziemann U, Rothwell JC (2000) I-waves in motor cortex. J Clin Neurophysiol 17(4):397–405
Acknowledgments
ARC-SRI: Science of Learning Research Centre (Project Number SR120300015).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horvath, J.C., Vogrin, S.J., Carter, O. et al. Effects of a common transcranial direct current stimulation (tDCS) protocol on motor evoked potentials found to be highly variable within individuals over 9 testing sessions. Exp Brain Res 234, 2629–2642 (2016). https://doi.org/10.1007/s00221-016-4667-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4667-8