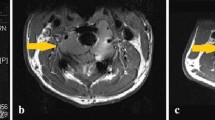
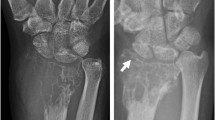
Abstract
Central giant cell granuloma (CGCG) is a rare lesion of the jaw occurring in young adults and adolescents. Surgery, the traditional mainstay of therapy, is associated with significant morbidity. Denosumab, a humanised monoclonal antibody to RANKL, is effective in a related entity, giant cell tumour of bone (GCTB), but experience in the more indolent CGCG is limited. This prospective observational study of all denosumab-treated CGCG at a tertiary referral centre (2015–2021) aimed to evaluate the safety, efficacy and recurrence risk using denosumab in CGCG at lower-frequency dosing than used for GCTB. All received standardised, time-limited courses of denosumab 120 mg with stepwise increase in dosing interval based on response. They were followed for up to 75 months using a radiation-minimising protocol: 3-monthly clinical, biochemical and radiological assessment (orthopantomograms, cone beam CT). Eight patients, median age 20.5 years [IQR 6], received 13 initial doses [IQR 10] of denosumab 120 mg. Radiologic response was seen after 5.5 doses [IQR 4.5]: ossification in all and size reduction in three. Recurrence occurred in four of seven completing therapy, observed 12 months post-cessation [IQR 6.5]. Larger baseline size, aggressive subtype and fewer than 12 initial doses were more common in the recurrence group. There was no osteonecrosis of the jaw. Hypocalcaemia occurred in one receiving modified dosing. This study represents the largest, most diverse cohort of denosumab-treated CGCG with the longest follow-up in literature. It demonstrates the efficacy of lower-frequency, time-restricted course of denosumab but highlights the risk of recurrence. Long-term follow-up is critical.







Similar content being viewed by others
References
Jaffe HL (1953) Giant-cell reparative granuloma, traumatic bone cysts, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 6(1):159–175. https://doi.org/10.1016/0030-4220(53)90151-0
de Lange J, van den Akker HP, van den Berg H (2007) Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104(5):603–615. https://doi.org/10.1016/j.tripleo.2007.04.003
Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A (1986) Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg 44(9):708–713. https://doi.org/10.1016/0278-2391(86)90040-6
Whitaker SB, Waldron CA (1993) Central giant cell lesions of the jaws. A clinical, radiologic, and histopathologic study. Oral Surg Oral Med Oral Pathol 5(2):199–208. https://doi.org/10.1016/0030-4220(93)90094-k
Chrcanovic BR, Gomes CC, Gomez RS (2018) Central giant cell lesion of the jaws: an updated analysis of 2770 cases reported in the literature. J Oral Pathol Med 47(8):731–739. https://doi.org/10.1111/jop.12730
Chrcanovic BR, Gomes CC, Dos Santos TR, Abreu MHNG, Gomez RS (2019) Clinical factors associated with the recurrence of central giant cell lesions. J Oral Pathol Med 48(9):799–802. https://doi.org/10.1111/jop.12937
Lee JC, Huang HY (2020) Soft tissue special issue: giant cell-rich lesions of the head and neck region. Head Neck Pathol 14(1):97–108. https://doi.org/10.1007/s12105-019-01086-2
Palmerini E, Picci P, Reichardt P, Downey G (2019) Malignancy in giant cell tumor of bone: a review of the literature. Technol Cancer Res Treat 18:1533033819840000. https://doi.org/10.1177/1533033819840000
Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M et al (2010) Denosumab in patients with giant cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 11(3):275–280. https://doi.org/10.1016/S1470-2045(10)70010-3
Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, Grimer RJ et al (2019) Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol 20(12):1719–1729. https://doi.org/10.1016/S1470-2045(19)30663-1
Lipplaa A, Dijkstra S, Gelderblom H (2019) Challenges of denosumab in giant cell tumor of bone, and other giant cell-rich tumors of bone. Curr Opin Oncol 31(4):329–335. https://doi.org/10.1097/CCO.0000000000000529
Gupta B, Stanton N, Coleman H, White C, Singh J (2015) A novel approach to the management of a central giant cell granuloma with denosumab: a case report and review of current treatments. J Craniomaxillofac Surg 43(7):1127–1132. https://doi.org/10.1016/j.jcms.2015.04.011
Hameed M, O’Connell JE, Rogers SN (2019) Management of an aggressive giant cell granuloma of the mandible with denosumab: a case report. Br J Oral Maxillofac Surg 57(7):691–693. https://doi.org/10.1016/j.bjoms.2019.05.023
Kim TS, Usera GL, Ruggiero SL, Weinerman SA (2017) Improvement of giant cell lesions of the jaw treated with high and low doses of denosumab: a case series. JMBR Plus. 1(2):101–106. https://doi.org/10.1002/jbm4.10010
Naidu A, Malmquist MP, Denham CA, Schow SR (2014) Management of central giant cell granuloma with subcutaneous denosumab therapy. J Oral Maxillofac Surg 72(12):2469–2484. https://doi.org/10.1016/j.joms.2014.06.456
Pogrel MA, Hossaini-Zadeh M (2021) Denosumab for the management of central giant cell granuloma of the jaws-a case series. Int J Oral Maxillofac Surg 50(8):1019–1022. https://doi.org/10.1016/j.ijom.2020.12.013
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Bredell M, Rordorf T, Kroiss S, Rucker M, Zweifel DF, Rostetter C (2018) Denosumab as a treatment alternative for central giant cell granuloma: a long-term retrospective cohort study. J Oral Maxillofac Surg 76(4):775–784. https://doi.org/10.1016/j.joms.2017.09.013
Schreuder WH, Coumou AW, Kessler PA, de Lange J (2014) Alternative pharmacologic therapy for aggressive central giant cell granuloma: denosumab. J Oral Maxillofac Surg 72(7):1301–1309. https://doi.org/10.1016/j.joms.2014.02.017
da Silva NG, Carreira AS, Pedreira EN, Tuji FM, Ortega KL, de Jesus Viana Pinheiro J (2012) Treatment of central giant cell lesions using bisphosphonates with intralesional corticosteroid injections. Head Face Med 8:23. https://doi.org/10.1186/1746-160X-8-23
Landesberg R, Eisig S, Fennoy I, Siris E (2009) Alternative indications for bisphosphonate therapy. J Oral Maxillofac Surg 67(5 Suppl):27–34. https://doi.org/10.1016/j.joms.2008.12.006
Gossai N, Hilgers MV, Polgreen LE, Greengard EG (2015) Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumour of bone. Pediatr Blood Cancer 62(6):1078–1080. https://doi.org/10.1002/pbc.25393
Marpuri I, Cheung CC, Moke DJ, Ryabets-Lienhard A (2021) Symptomatic rebound hypercalcemia after denosumab discontinuation in a pediatric patient with cherubism. J Endocr Soc. 5(S1):A706-707. https://doi.org/10.1210/jendso/bvab048.1439
Huang B, Law MW, Khong PL (2009) Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology 251(1):166–174. https://doi.org/10.1148/radiol.2511081300
Ludlow JB, Davies-Ludlow LE, White SC (2008) Patient risk related to common dental radiographic examinations: the impact of 2007 international commission on radiological protection recommendations regarding dose calculation. J Am Dent Assoc 139(9):1237–1243. https://doi.org/10.14219/jada.archive.2008.0339
Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ (2001) Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics 21(5):1283–1309. https://doi.org/10.1148/radiographics.21.5.g01se251283
Edwards PC (2015) Insight into the pathogenesis and nature of central giant cell lesions of the jaws. Med Oral Patol Oral Cir Bucal 20(2):e196-198. https://doi.org/10.4317/medoral.20499
Gomes CC, Gayden T, Bajic A, Harraz OF, Pratt J, Nikbakht H, Bareke E et al (2018) TRPV4 and KRAS and FGFR1 gain-of-function mutations drive giant cell lesions of the jaw. Nat Commun 9(1):4572. https://doi.org/10.1038/s41467-018-06690-4
Funding
No funding, grants or other support relevant to this manuscript was received.
Author information
Authors and Affiliations
Contributions
YJR is responsible for the overall content. CMG, YJR, CFM and LL contributed to the study conception and design. Material preparation and data collection were performed by YJR, CJW, MN, JAV, HC, JK, LL, DJH and CMG. Data analysis was performed by YJR and CMG. The first draft of the manuscript was written by YJR, CMG and DJH, and all authors commented on and revised each version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Craig F. Munns has previously been involved in an Amgen-sponsored clinical trial (not related to this manuscript). Christian M. Girgis has received an honorarium from Amgen for educational presentations on romosozumab. Yoon Ji Jina Rhou, Che-Jen Wang, Minh Nguyen, Joel A. Vanderniet, Craig F. Munns, Hedley Coleman, James Kim, Deborah Jane Holmes-Walker, Lydia Lim, and Christian M. Girgis have no other relevant financial or non-financial conflicts of interest to declare.
Human and Animal Rights and Informed Consent statement
Ethics approval was obtained from the Western Sydney Local Health District Human Ethics Committee and procedures followed were in accordance with the ethics standards of the committee and with the Helsinki Declaration. All data was collected as part of the participants' routine clinical management. Informed consent was obtained prior to photographing and the research participant has provided informed consent for publication of the images in Figures 4a and 4b.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rhou, Y.J.J., Wang, CJ., Nguyen, M. et al. Clinical and Radiologic Response of Central Giant Cell Granuloma to Denosumab: A 6-Year Prospective Observational Study. Calcif Tissue Int 110, 464–474 (2022). https://doi.org/10.1007/s00223-021-00935-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00935-z