Abstract
Background
Serious adverse effects have been observed with some non-sedative H1-antihistamines (terfenadine and astemizole) when they were associated with drugs known to inhibit their metabolism. However, this is not a class effect, and this interaction should be considered on a case-by-case basis. The aim of this study was to evaluate the potential of pharmacokinetic interaction between cetirizine and ritonavir, the most potent cytochrome P 450 (CYP) inhibitor.
Methods
An open-label, single-center, one-sequence crossover pharmacokinetic study was conducted in three running periods: cetirizine (CTZ) alone, ritonavir (RTV) alone and then CTZ plus RTV. For each period, steady-state pharmacokinetics were obtained. RTV and CTZ plasma concentrations were determined using validated liquid chromatography methods. The statistical method was based on a 90% confidence interval (CI) for the ratio of population geometric means (combination/drug alone) for each drug and for each parameter [area under the plasma concentration versus time curve (AUC0-τ,ss), value of maximum plasma concentration (Cmax,ss)] and compared to bioequivalence ranges 80–125% and 70–143% for AUC0-τ,ss and Cmax,ss, respectively.
Results
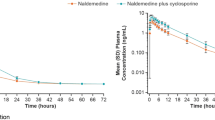
Among the 17 male subjects enrolled (26.4±8.6 years), 16 completed the study (1 withdrawal after the first period). The RTV pharmacokinetic parameter values were not affected by CTZ co-treatment. With RTV, a 42% increase in the CTZ AUC0-τ,ss (3406 versus 4840 μgh/l, 90% CI of 128–158%), a 53% increase in the CTZ elimination half-life (7.8 h versus 11.9 h, P = 0.001), a slight increase (15%) in the CTZ apparent volume of distribution (Vd,ss/ f) (34.7 l versus 39.8 l, P = 0.035), a 29% decrease in the CTZ apparent total body clearance (49.9 ml/min versus 35.3 ml/min, P<0.001) and bioequivalent Cmax,ss (374 μg/l versus 408 μg/l) were observed. No serious drug related adverse effects were notified.
Conclusions
CTZ does not significantly affect the pharmacokinetic parameters of RTV, and the association does not, thus, require a modification of the dosage of the protease inhibitor. The increased extent of exposure to CTZ in healthy subjects, in the presence of RTV administered at high doses, remained in the same range as previously observed in the elderly or in mildly renally impaired subjects.
Similar content being viewed by others
References
Snyder S, Snowman AM (1987) Receptor effects of cetirizine. Ann Allergy 59:4–8
Gillard M, Van der Perren C, Moguilevsky N, Massingham R, Chatelain P (2002) Binding characteristics of cetirizine and levocetirizine to human H1 histamine receptors: contribution of Lys191 and Thr194. Mol Pharmacol 61:1–9
Wood SG, John BA, Chasseud LF, Yeh J, Chung M (1987) The metabolism and pharmacokinetics of 14 C-cetirizine in humans. Ann Allergy 59:31–34
Whomsley R, Collart P, Strolin Benedetti M, Baltes E, Nicolas JM (2003) Stereoselectivity of cetirizine metabolism. Drug Metab Rev 35(1):30
Matzke GR, Yeh J, Awni WM, Halstenson CE, Chung M (1987) Pharmacokinetics of cetirizine in the elderly and patients with renal insufficiency. Ann Allergy 59:25–30
Horsmans Y, Desager JP, Hulhoven R, Harvengst C (1993) Single-dose pharmacokinetics of cetirizine in patients with chronic liver disease. J Clin Pharmacol 33:929–932
Tillement JP (1995) A low distribution volume as a determinant of efficacy and safety for histamine (H1) antagonists. Allergy 50:12–16
Kim RB (2002) Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev 34:47–54
Perloff MD, von Moltke LL, Greenblatt DJ (2002) Fexofenadine transport in Caco-2 cells: inhibition with verapamil and ritonavir. J Clin Pharmacol 42:1269–1274
Whomsley R, Gerin B, Brochot A, Strolin Benedetti M, Baltes E (2003) Transport characteristics of cetirizine and levocetirizine in Caco-2 cell monolayers. Allergy 58(74):274–275
Chen C, Hanson E, Watson JW, Lee JS (2003) P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab Dispos 31:312–318
Hsu A, Granneman GR, Bertz RJ (1998) Ritonavir Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet 35(4):275–291
Kumar GN, Rodrigues A, Buko AM, Denissen JF (1996) Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther 77:423–431
Denissen JF, Grabowski BA, Johnson MK, Buko AM, Kempf DJ, Thomas SB, Surber BW (1997) Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs and humans. Drug Metab Dispos 25:489–501
Hsu A, Granneman R, Witt G, Locke C, Denissen J, Akhteruzzaman M et al (1997) Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother 41(5):898–905
Barry M, Gibbons S, Back D, Malcahy F (1997) Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations. Clin Pharmacokinet 32(3):194–209
NORVIR Ritonavir (2001) Summary of product characteristics. Abbott Laboratories
Fleiss JL (1986) The design and analysis of clinical experiments. Wiley Interscience, New York
Dricot E, Jeanbaptiste B, Stockis A. Validation of a LC method using MS/MS detection for the determination of cetirizine and its metabolite ucb P026 in human plasma. UCB Study TA0447. SGS Biopharma analytical report No. 197.594/1
Marsh KC, Eiden E, McDonald E (1997) Determination of ritonavir, a new HIV protease inhibitor, in biological samples using reversed-phase high-performance liquid chromatography. J Chromatogr 704:307–313
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York
Endrenyi L, Yan W (1993) Variation of C max /AUC in investigations of bioequivalence. Int J Clin Pharmacol Ther Toxicol 31(4):184–189
Chung M (1984) Computation of model-independent pharmacokinetic parameters during multiple dosing. J Pharm Sci 73:570–571
Chow SC, Liu JP (1992) Design and analysis of bioavailability and bioequivalence studies. Marcel Dekker, New York
Lehmann EL (1975) Nonparametrics: Statistical methods based on ranks. McGraw-Hill International Book Company, New York, p 436
Hauschke D, Steinijans VW, Diletti E (1990) A distribution-free procedure for the statistical analysis of bioequivalence studies, Int J Clin Pharmacol Ther Toxicol 28:72–78
Sale M, Woosley R, Thakker K, Phillips K, Caridi F, Chung M (1996) Effects of cetirizine and erythromycin, alone and in combination, on QT interval and pharmacokinetics in healthy subjects. Ann Allergy Asthma Immunol 74:93
Ouellet D, Hsu A, Qian J, Lamm JE, Cavanaugh JH, Leonard JM, Granneman GR (1998) Effect of fluoxetine on pharmacokinetics of ritonavir. Antimicrob Agents Chemother 42:3107–3112
Diletti E, Hauschke D, Steinijans VW (1991) Sample size determinations for bioequivalence assessment by means of confidence intervals. Int J Clin Pharmacol Ther Toxicol 29(1):1–8
Lin JH, Yamazaki M (2003) Role of P-glycoprotein in pharmacokinetics. Clin Pharmacokinet 42:59–98
Hansten PD, Levy RH (2001) Role of P-glycoprotein and organic anion transporting polypeptides in drug absorption and distribution. Focus on H1 receptor antagonists. Clin Drug Invest 21(8):587–596
Mulato AS, Ho ES, Cihlar T (2000) Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther 295(1):10–15
Ding R, Tayrouz Y, Riedel KD, Burhenne J, Weiss J, Mikus G, Haefeli WE (2004) Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther 76:73–84
Acknowledgements
The authors thank C. De Vos for her role in instigating this study, E. Baltes for useful discussion and M. Rovei for carefully checking the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peytavin, G., Gautran, C., Otoul, C. et al. Evaluation of pharmacokinetic interaction between cetirizine and ritonavir, an HIV-1 protease inhibitor, in healthy male volunteers. Eur J Clin Pharmacol 61, 267–273 (2005). https://doi.org/10.1007/s00228-005-0917-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0917-6