Abstract
Objective
To determine the effect of artesunate (AT) on the disposition kinetics of sulfadoxine/pyrimethamine (SP) in humans.
Methods
In a randomized cross-over study, 16 healthy volunteers were given a dose of three SP tablets containing 500 mg of sulfadoxine (SDX) and 25 mg of pyrimethamine (PYR) (=SP group), while the second arm received three SP tablets + two AT tablets of 200 mg in total followed by 100 mg AT for the next 4 days (SP+AT group). Blood samples (100 μl) were collected by means of a finger prick and dried on filter paper. The blood spots were wrapped in polythene folders and stored at room temperature until analysis. The samples were assayed using high-performance liquid chromatographic methods.
Results
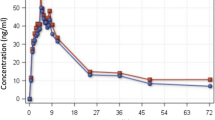
The peak concentration Cmax), time required to attain peak concentration (Tmax), half-life (t ½) and area under the plasma concentration-time curve (AUC) were determined. The Cmax of SDX were 92.9 and 98.9 μg/ml for the SP and SP+AT arms, respectively; for PYR, these were 0.86 and 0.79 μg/ml, respectively. The Tmax of SDX were 10 and 8 h for the SP and SP+AT arms, respectively; for PYR, these were 4.0 and 3.0 h, respectively. The AUC0–288 of SDX were 15,840 and 18,876 μg/ml h for the SP and SP+AT arms, respectively; for PYR, they were 124 and 112 μg/ml h, respectively. The t ½ of values for SDX were 165 and 180 h for the SP and SP+AT arms, respectively; for PYR, these were 158 and 177 h, respectively. There was no statistically significant difference between the Cmax, Tmax, AUC0–288 and t ½ between the two arms (p > 0.05).
Conclusion
Taking AT concomitantly with SP does not have any impact in the disposition of SP.

Similar content being viewed by others
References
Taylor WR, Rigal J, Olliaro PL (2003) Drug resistant falciparum malaria and the use of artesunate-based combinations: focus on clinical trials sponsored by TDR. J Vector Borne Dis 40:65–72
Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA, Estrada-Franco JG, Mollinedo RE, Avila JC, Cespedes JL, CATer D, Doumbo OK (1997) Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic pattern of pyrimethamine-sulfadoxine use and resistance. J Infect Dis 176:1590–1596
Schneider P, Bousema T, Omar S, Gouagna L, Sawa P, Schallig H, Sauerwein R (2006) Submicroscopic Plasmodium falciparum gametocytaemia in Kenyan children after treatment with sulphadoxine-pyrimethamine monotherapy or in combination with artesunate. Int J Parasitol 36:403–408
Mugitu K, Abdulla S, Falk N, Masanja H, FElger I, Mshinda H, Beck Hp, Genton B (2005) Efficacy of sulphadoxine-pyrimethamine in Tanzania after two years as first-line drug for treatment of uncomplicated malaria: assessment protocol and implication of treatment policy strategies. Malar J 4:55
Mubyazi GM (2003) The role of Research in changing the first-line antimalarial drug policy in Tanzania. Working Paper. Alliance for Health Policy and Systems Research (AHPSR), Geneva
Majori G (2004) Combined antimalarial therapy using artemisinin. Parassitologia 46:85–87
Bloland PB (2000) Combination therapy for malaria in Africa: hype or hope? Bull World Health Organ 78:1378–1388
Coleman PG, Morel C, Shillcutt S et al (2004) A threshold analysis of the cost-effectiveness of ACTs in sub-Saharan Africa. Am J Trop Med Hyg 71:196–240
Olliaro PL, Taylor WR (2004) Developing artemisinin based drug combinations for the treatment of drug resistant falciparum malaria: A review. J Postgrad Med 50:40–44
Ribera E, Pou L, Lopez RM, Crespo M, Falco V, Ocana I, Ruiz I, Pahissa A (2001) Pharmacokinetic interaction between nevirapine and rifampicin in HIV-infected patients with tuberculosis. J Acquir Immune Defic Syndr 28:450–453
Ridtitid W, Wongnawa M, Mahatthanatrakul W, Raungsri N, Sunbhanich M (2005) Ketoconazole increases plasma concentrations of mefloquine in healthy human. J Clin Pharm Ther 30:285–290
Ridtitid W, Wongnawa M, Mahatthanatrakul W, Punyo J, Sunbhanich M (2002) Rifampin markedly decreases plasma concentrations of praziquantel in healthy volunteers. Clin Pharmacol Ther 72:505–513
Karbwang J, Na Bangchang K, Thanavibul A, Back DJ, Bunnag D, Harinasuta T (1994) Pharmacokinetics of mefloquine alone or in combination with artesunate. Bull World Health Organ 72:83–87
Elamin SB, Malik EM, Abdelgadir T, Khamiss AH, Mohammed MM, Ahmed ES, Adam I (2005) Artesunate plus sulfadoxine-pyrimethamine for treatment of uncomplicated Plasmodium falciparum malaria in Sudan. Malar J J 4:41
Priotto G, Kabakyenga J, Pinoges L, Ruiz A, Eriksson T, Coussement F, Ngambe T, Taylor WR, Perea W, Guthmann JP, Olliaro P, Legros D (2003) Artesunate and sulfadoxine-pyrimethamine combinations for the treatment of uncomplicated Plasmodium falciparum malaria in Uganda: a randomized, double-blind, placebo-controlled trial. Trans R Soc Trop Med Hyg 97:325–330
Obonyo CO, Ochieng F, Taylor WR, Ochora SA, Mugitu K, Olliaro P, Ter kiile F, Oloo AJ (2003) Artesunate plus sulfadoxine-pyrimethamine for uncomplicated malaria in Kenyan children: a randomized, double-blind, placebo-controlled trial. Trans R Soc Trop Med Hyg 97:585–591
von Seidlein L, Milligan P, Pinder M, Bojang K, Anyalebechi C, Gosling R, Coleman R, Ude JI, Sadiq A, Duraisingh M, Warhurst D, Alloueche A, Targett G, McAdam K, Greenwood B, Walraven G, Olliaro P, Doherty T (2000) Efficacy of artesunate plus pyrimethamine-sulphadoxine for uncomplicated malaria in Gambian children: a double-blind, randomised, controlled trial. Lancet 355:2080
Tjitra E, Suprianto S, Currie BJ, Morris PS, Saunders JR, Anstey NM (2001) Therapy of uncomplicated falciparum malaria: a randomized trial comparing artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone in Irian Jaya, Indonesia. Am J Trop Med Hyg 65:309–317
Marquino W, Ylquimiche L, Hermenegildo Y, Palacios AM et al (2005) Efficacy and tolerability of artesunate plus sulfadoxine-pyrimethamine and sulfadoxine-pyrimethamine alone for the treatment of uncomplicated Plasmodium falciparum malaria in Peru. Am J Trop Med Hyg 72:568–572
Lilja JJ, Kivisto KT, Neuvonen PJ (2000) Duration of effect of grapefruit Juice on the pharmacokinetics of the CYP3A4 substrate simvastatin. Clin Pharmacol Ther 68:384–390
Bergqvist Y, Hjelm E, Rombo L (1987) Sulphadoxine assay using capillary blood samples dried on filter paper-suitable for monitoring of blood concentration in the field. Ther Drug Monit 9:203–207
Minzi OM, Massele AY, Gustafsson LL, Ericsson O ( 2005) Simple and cost-effective liquid chromatographic method for determination of pyrimethamine in whole blood samples dried on filter paper. J Chromatogr B Anal Technol Biomed Life Sci 814:179–183
Svensson US, Ashton M (1999) Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol 48:528–35
Zielinska E, Niewiarowski W, Bodalski J, Stanczyk A, Bolanowski W, Rebowski G (1997) Arylamine N-acetyltransferase (NAT2) gene mutations in children with allergic diseases. Clin Pharmacol Ther 62:635–642
Chanda P, Hawela M, Kango M, Sipilanyambe N (2006) Assessment of the therapeutic efficacy of a paediatric formulation of artemether-lumefantrine (Coartesiane) for the treatment of uncomplicated Plasmodium falciparum in children in Zambia. Malar J 5:75
Price RN, Nosten F, Luxemburger C, van Vugt M, Phaipun L, Chongsuphajaisiddhi T, White NJ (1997) Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg 91:574–577
Adam I, Magzoub M, Osman ME, Khalil IF, Alifrangis M, Elmardi KA (2006) A fixed-dose 24-hour regimen of artesunate plus sulfamethoxypyrazine-pyrimethamine for the treatment of uncomplicated Plasmodium falciparum malaria in eastern Sudan. Ann Clin Microbiol Antimicrob 5:18–22
Acknowledgements
This study was financially supported by the Swedish Agency for Research Cooperation with Developing Countries (SAREC), through grant no. SWE-2000-175, SWE-BIL-96, and is gratefully acknowledged. The Sumaria Group Ltd in Tanzania also provided additional funding for the study. Mr. Bwile Paschal ran the HPLC sample analyses whereas Dr. Mary Jande, Mr. Jumanne Mng’agi, Mr. James Fulgence and Mr. Walter Msangi gave technical support. The Division of Clinical Pharmacology at Kalorinska Institute in Stockholm., donated the pharmacokinetic software. This study would not have been possible without the participation of volunteers. We highly thank them for committing their time and willingness to participate in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minzi, O.M.S., Gupta, A., Haule, A.F. et al. Lack of impact of artesunate on the disposition kinetics of sulfadoxine/pyrimethamine when the two drugs are concomitantly administered. Eur J Clin Pharmacol 63, 457–462 (2007). https://doi.org/10.1007/s00228-007-0278-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0278-4